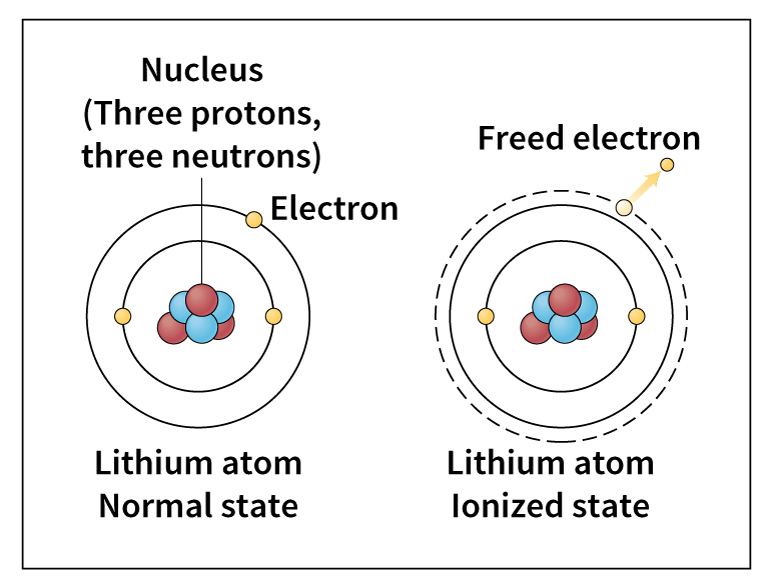
Ion << EYE uhn or EYE on >> is an atom or molecule that has an electric charge. Atoms and molecules become charged if they gain or lose electrons. Each atom has a cloud of negatively charged electrons around a small, heavy nucleus. The nucleus contains positively charged protons. If the number of electrons around the nucleus equals the number of protons inside the nucleus, the atom is neutral. The process of removing electrons from atoms or molecules to produce positive ions is called ionization. The electrons removed may then join other atoms or groups of atoms, causing them to become negative ions. The amount of electric charge an ion has is determined by the number of electrons gained or lost by the atom or molecule.
Many common substances contain ions. For example, table salt consists of equal numbers of positively charged sodium ions and negatively charged chloride ions. In forming table salt from the elements sodium and chlorine, each sodium atom loses an electron and becomes a positive sodium ion. Likewise, each chlorine atom gains an electron and becomes a negative chloride ion. Seawater contains many kinds of ions. The most common ones, in order of their amount, are chloride, sodium, sulfate, and magnesium. The earth’s atmosphere also contains ions. They are concentrated in a layer called the ionosphere.
Ions in solids
usually fit together in a regular, repeating, three-dimensional arrangement. Such a substance is called an ionic crystal. For example, sodium ions alternate with chloride ions in a crystal of table salt. Ions in an ionic crystal are held together by electrostatic attraction, the attraction between opposite charges.
Ions in liquids
can migrate throughout the liquid. In a solution, each ion attracts one or more molecules of the solvent (dissolving liquid). Ionic crystals, such as sodium chloride, usually dissolve only in solvents that contain polar molecules. Polar molecules have a positive end and a negative end. Each ion on the surface of the crystal attracts the oppositely charged end of polar molecules. This attraction weakens the attraction between ions in the crystal. The ions then break away from the crystal and enter the solution, surrounded by polar molecules. Ions combined with polar molecules of the solvent are said to be solvated.
Ions in gases
are too far apart at normal pressures to attract each other strongly. As a result, single ions in a gas may drift for a long time before they combine with other ions. A mixture consisting of ionized gas and electrons is called a plasma.
Many particles in space are ions. Some of these ions are trapped by the earth’s magnetic field and make up part of the Van Allen belts.
Behavior of ions.
All ionic solids and liquids, and most ionized gases, are electrically neutral. The total charge of all their positive ions equals the total charge of all their negative ions. This general rule also applies to all other kinds of matter, and is called the principle of electroneutrality.
Like neutral atoms and molecules, ions in a liquid or a gas are constantly moving. Each one changes its direction of motion billions of times each second because of collisions and the forces exerted on it by other particles. After each change in direction, an ion usually is no more likely to be moving in one direction than in any other. Such random motion is called Brownian motion. When two oppositely charged conductors called electrodes are placed in a liquid or gas, each ion loses part of its random motion and starts to drift toward one of the electrodes. Negative ions, called anions, move toward one electrode—called the anode—where they lose electrons. Positive ions, called cations, move toward the other electrode—called the cathode—where they gain electrons. The movement of the charges carried by the moving ions in solution makes up an electric current.
The ability of a solution to conduct electric current depends on the concentration of ions in the solution. For example, drinking water drawn from a typical municipal treatment plant in the United States contains few ions and therefore is a poor conductor of current. But seawater, with significant amounts of dissolved sodium chloride, magnesium sulfate, and other ionic compounds, is a good conductor.
Producing ions.
Any process that can add or remove electrons from an atom or a molecule can produce ions. Radiation and chemical reactions are such processes. Radiation can increase the energy of the electrons in an atom. If this energy is increased enough, one or more electrons can overcome the attraction of the nucleus and escape from the atom. The atom thereby becomes a positive ion. Radiation that can produce ions includes ultraviolet rays, X rays, gamma rays, atomic nuclei, subnuclear particles, and electrons.
High-energy radiation absorbed by plant or animal tissues produces unnatural ions in the tissues. These ions become involved in potentially harmful chemical reactions. In human beings and other animals, the symptoms of these reactions are called radiation sickness. The amount of radiation absorbed by tissues is measured in rads. One rad corresponds to the formation of more than a billion pairs of ions in the tissues. Death is likely to occur if a person absorbs about 500 rads over a short period. In the metric system, the unit of absorbed dose is the gray, which equals 100 rads.
Ions are formed in a chemical reaction if molecules split into electrically charged parts. For example, molecules of sodium chloride (NaCl) split when added to water. They form positive sodium ions and negative chloride ions.
