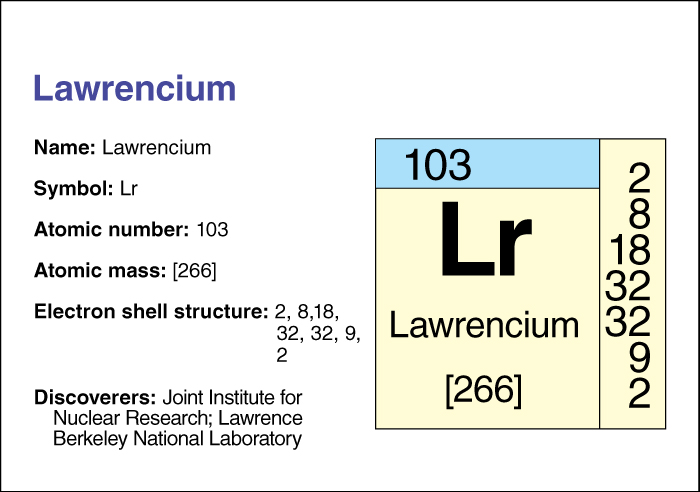
Lawrencium is an artificially produced radioactive chemical element . It has an atomic number (number of protons) of 103. Its chemical symbol is Lr. The most stable isotope (form) of lawrencium has an atomic mass number of 266. An isotope’s atomic mass number is equal to the total number of protons and neutrons in the nucleus (core) of an atom of that isotope. Isotope 266 has a half-life of 11 hours—that is, due to radioactive decay, only half the atoms in a sample of isotope 266 would still be atoms of that isotope after 11 hours.
Chemists classify lawrencium in the actinide group among the transuranium elements . For information on the position of lawrencium on the periodic table, see the article Periodic table .
In 1961, scientists at Lawrence Radiation Laboratory (now Lawrence Berkeley National Laboratory ) in Berkeley, California, claimed that they had produced an element with an atomic number of 103. They had bombarded the element californium , which has an atomic number of 98, with boron , atomic number 5. In 1965, scientists at the Joint Institute for Nuclear Research in Dubna, Russia, near Moscow , made a rival claim. From 1968 to 1971, both the Berkeley and Dubna groups offered additional evidence for the existence of element 103.
The Berkeley group proposed the name lawrencium to honor the American physicist Ernest O. Lawrence . This name was accepted by the International Union of Pure and Applied Chemistry (IUPAC). The IUPAC is the recognized authority in crediting the discovery of elements and assigning names to them. In 1986, the IUPAC and the International Union of Pure and Applied Physics formed a working group to review the histories of the elements with atomic numbers from 101 to 109. In 1993, the IUPAC accepted the group’s decision that the discovery of lawrencium was a cumulative effect of work done at Berkeley and Dubna from 1961 to 1971.
Until 2014, the most stable isotope of lawrencium known was number 262, with a half-life of 3.6 hours. In that year, scientists working to produce element 117 (now known as tennessine ) announced the discovery of a longer-lived lawrencium 266 isotope, with four additional neutrons, as one of the decay products of their reaction. The 11-hour half-life of lawrencium 266 makes it the longest-lived isotope of this element.
