Light is a form of energy so common that we often take it for granted. Yet it is difficult to imagine what our world would be like without light. The light that enters our eyes causes a chain of chemical reactions that make seeing possible. Without light, therefore, we could not see. Without the energy from light, we would not have the food we eat or the air we breathe. Green plants and other organisms, such as algae, use light from the sun to grow and to make food through a process called photosynthesis. The food we eat comes from these photosynthetic organisms or from animals, fungi, and other living things that depend on them for food. As photosynthetic organisms grow, they give off oxygen, a necessary part of the air we breathe.

The energy in fuels also comes from light. Plants and other organisms living millions of years ago stored the energy of sunlight that fell on Earth. Over time, buried plant life and other once-living things decayed and became fossil fuels—coal, natural gas, and oil. Since the 1800’s, we have used the energy in these fuels to produce electric power and to operate machines.
People have found ways of making and controlling light, enabling them to see when there is no sunlight. At first, they produced light with fire—using campfires, torches, and later candles and oil lamps. In the late 1800’s, they developed electric light bulbs. In the mid-1900’s, scientists developed tiny and efficient light sources called light-emitting diodes (LED’s).

People make and use light for many purposes besides seeing. For example, a home entertainment system uses several light sources. An LED sends signals from a remote control to a DVD player. The player uses a light source called a laser to read the movie from the DVD. A third source generates the image on a television screen.
Using scientific instruments, people can study light and learn much about the universe. Astronomers examine the light from distant stars to determine what atoms the stars contain. This light can also reveal if the stars are moving toward or away from Earth and how fast they are moving.
What is light? People once thought light was something that traveled from a person’s eyes to an object and then back again. Ancient scholars pictured light as traveling in rays. We now understand that light is a form of electromagnetic energy that can travel freely through space. This energy travels in patterns of electric and magnetic influence called electromagnetic waves. There are many kinds of electromagnetic energy, including infrared rays, radio waves, ultraviolet rays, and X rays. Human beings can see only a tiny part of all the different kinds of electromagnetic energy. This part is called visible light.
This article describes where light comes from, the nature of light, and how light behaves. The article also tells how light is measured and discusses important uses of light in technology. For more information on how people use light for seeing, see Lighting.
Sources of light
Many of the things we see, including the sun and light bulbs, are sources of light. We can see them because they give off light that travels directly to our eyes. Other objects, such as the moon or our bodies, give off no visible light. We can see these objects because light from a source bounces off them before reaching our eyes. Light sources that we do not control, including the sun and other stars, are called natural sources. Sources made by human beings, or artificial sources, include such objects as candles and streetlights.
How light is produced.
Most light that we see comes from electrons within atoms. An electron is an extremely small particle with a tiny, negative electric charge. Light is produced by electrons that have gained energy, either by absorbing light from another source or by being struck by other particles. An atom with such an electron is said to be excited. Ordinarily, an atom stays excited briefly. It de-excites by giving up the extra energy. Either it can transfer the energy to another atom in a collision, or its electrons can emit (give off) visible light or another kind of electromagnetic energy. The light carries away the extra energy. Different kinds of atoms require different amounts of energy to excite them, and they emit different amounts of energy as light.
An electron can emit light even if it is free (not part of an atom). An accelerating or decelerating free electron will give off electromagnetic energy, losing some of its own energy in the process. Scientists can pass electrons traveling at high speeds through a series of magnets arranged with alternating poles. The alternating magnetic forces “shake” the electrons, causing them to accelerate and decelerate in regular cycles. As a result, the electrons emit electromagnetic waves with a distinct frequency. A wave’s frequency is the number of individual waves or cycles that pass a given point in a certain period. Scientists can use these devices, called free electron lasers, to produce a narrow beam of laserlike light.
Much light is generated by high-speed particles colliding with atoms. Inside the core of the sun, for example, nuclear reactions produce energetic particles that collide with atoms at the sun’s surface. These atoms give off the sunlight that falls on Earth. All stars emit light by this process during much of their lives.
Vibrating molecules can also produce light. Within a molecule, the forces that hold atoms together act like springs. An impact can leave the atoms vibrating with respect to one another like a tuning fork that vibrates after being struck. Just as a tuning fork gives off energy to the air in the form of sound, a vibrating molecule can release energy by emitting light.
Large eruptions on the sun’s surface produce high-speed particles that crash into the atoms and molecules of Earth’s atmosphere. These collisions excite the atoms and molecules of the atmosphere with extra energy. The atoms and molecules then release the energy in a display of light called an aurora.
When light strikes an object, the object absorbs the light in small units of electromagnetic energy called photons. The energy of a photon, along with the color of the light, depends on its frequency. High-energy photons have higher frequencies and bluer colors. Low-energy photons have lower frequencies and redder colors. Thus, we can think of the light in a rainbow as spanning a range of energies—from low energy, or red, to high energy, or violet.
Incandescence.
One way to excite atoms to emit light is by heating them. The process by which a heated object gives off light is called incandescence.
In a traditional incandescent light bulb, electric current passes through a filament (wire). The electrons of the current collide violently with one another and with the atoms of the filament, heating the filament and exciting the atoms. As a result, the atoms emit a stream of light with a wide range of energies. The combination of all the resulting colors is white, and the filament glows white hot. As the filament cools, fewer atoms are excited to high energies, and the atoms emit fewer photons with the higher energies of blue light. Because red light is still being emitted, the cooler filament glows red.
Luminescence and fluorescence.
Many substances gain energy and emit light without being heated much. They do this through a process called luminescence.
Some luminescent materials glow in the dark long after they have received extra energy. They are said to be phosphorescent. Manufacturers use certain phosphorescent materials to make glow-in-the-dark toys.
Certain life forms can phosphoresce, an ability called bioluminescence. In this process, chemicals within the organisms combine to produce a different chemical that has excited atoms. When the atoms de-excite, they emit photons. Such organisms can often be seen in breaking waves near the seashore at night. 
Other luminescent materials emit light only during their exposure to exciting energy. They are said to be fluorescent. Fluorescence occurs when a material absorbs high-energy photons, typically ultraviolet light, and emits lower-energy photons, typically visible light. A fluorescent material may reflect one color of light and emit a different color. For example, a material that appears green under white light could absorb ultraviolet photons from a black light and then emit yellow light.
Some materials emit light when exposed to an electric current or voltage, such as that produced by a battery. This type of light emission is called electroluminescence. Manufacturers use electroluminescent materials for making LED’s and for backlighting such devices as alarm clocks and digital music players.
The spectrum of light sources.
When sunlight is viewed through a prism, the light separates into the colors of the rainbow. A prism is a specially shaped transparent object typically made of glass or plastic. From red to violet, the colors blend from one to the next, forming an unbroken band called a continuous spectrum. A spectrum (plural spectra) is a band of light arranged in order of frequency. The collection of colors that we can see makes up the visible spectrum.
Each color has a frequency and a wavelength, the distance in space occupied by a single cycle. The speed of light equals the product of the frequency and the wavelength. Physicists express this fact with the equation c = f x l. In this equation, c stands for the speed of light, f stands for the frequency of the light wave or photon, and l stands for its wavelength. Because the speed of light in empty space is always the same, this equation yields a simple relationship between frequency and wavelength: the higher the frequency, the shorter the wavelength.
The visible spectrum forms only a small part of the full range of light waves. Green light, which lies at the center of the visible spectrum, has wavelengths of about 500 nanometers, or less than 1 percent of the diameter of a human hair. A nanometer is a billionth of a meter, or about 1/25,400,000 inch. Waves that have wavelengths slightly shorter than those of visible light are called ultraviolet rays. Ultraviolet light from the sun can cause sunburns and skin cancer, but many forms of ultraviolet light find use in medicine and technology. Waves with somewhat shorter wavelengths than ultraviolet rays are called X rays. These rays can penetrate a person’s body. Doctors and dentists use them to “see” inside the body. Gamma rays have even shorter wavelengths than X rays. They result from nuclear reactions, such as those in the sun. Light with wavelengths slightly longer than those of red light is called infrared light. When you stand in bright sunlight or in front of a fire, your skin feels warm mainly because of the infrared light shining on it.
Many sources of light do not produce a continuous spectrum. For example, a street lamp may give off bright yellow, blue, and a few other colors of light. Each color comes from certain atoms of the gas inside the lamp. The yellow, for example, comes from sodium atoms. Each type of atom can produce only certain colors, so the lamp will have dark regions in its spectrum.
Scientists can learn what kinds of atoms make up a light source by observing what colors are present in its spectrum. They direct the light through an instrument called a spectrometer to separate the colors. The spectrometer may be a simple prism or a more complicated device. Scientists can use a spectrometer with telescopes and other imaging devices to understand what types of atoms are contained in distant stars and in the outer atmosphere of the sun.
Our understanding of light
For many centuries, people have tried to explain what light is and why it acts as it does. As scientists gained knowledge of the physical world, they were led to reconsider their understanding of light. In turn, efforts to understand light and its effects have inspired scientists to create new ideas about how the world works.
Early ideas.
In most observations with the unaided eye, light appears to travel along a thin, straight line. Scientists call such a line a ray. When a light ray meets an object, the material can block, absorb, or bend the ray. In the early 1000’s, the Arab scientist and mathematician Alhazen wrote a book containing a detailed theory on the properties of light rays and their role in vision. For this work, Alhazen is sometimes known as the founder of optics, the study of light and vision. Although the idea of light as a ray is incomplete, in many situations it can help us understand why light travels along a certain path. In fact, scientists have designed and built sophisticated optical systems using only the ray model of light.
Modern study
of light began in the 1600’s. In 1666, the English scientist Sir Isaac Newton discovered that white light consists of all colors. Using a prism, he found that each color in a beam of white light could be separated. Newton proposed the theory that light consists of tiny particles that travel along rays. He called these particles corpuscles, and his theory became known as the corpuscular theory.
About the same time that Newton proposed his theory of light, the Dutch physicist and astronomer Christiaan Huygens suggested that light consists of waves. For nearly 100 years, scientists argued about whether light was a particle or a wave. Then, in the early 1800’s, the English physicist Thomas Young showed that two light beams can add to or cancel each other under certain conditions, an effect called interference. Scientists could explain this observation only by assuming that light was a wave.
The properties of waves.
To understand the wavelike behavior of light, we can start by thinking about more familiar types of waves. For example, some water waves travel along the water’s surface, raising and lowering the water as they pass. These waves have vertical crests (high points) and troughs (low points), but they travel in a horizontal direction along a ray. Because the water moves at right angles to the direction in which the waves travel, the waves are called transverse waves. Rapidly shaking the loose end of a rope can produce transverse waves as well. The rope will oscillate (move back and forth) while the wave travels along its length, carrying energy to the other end. 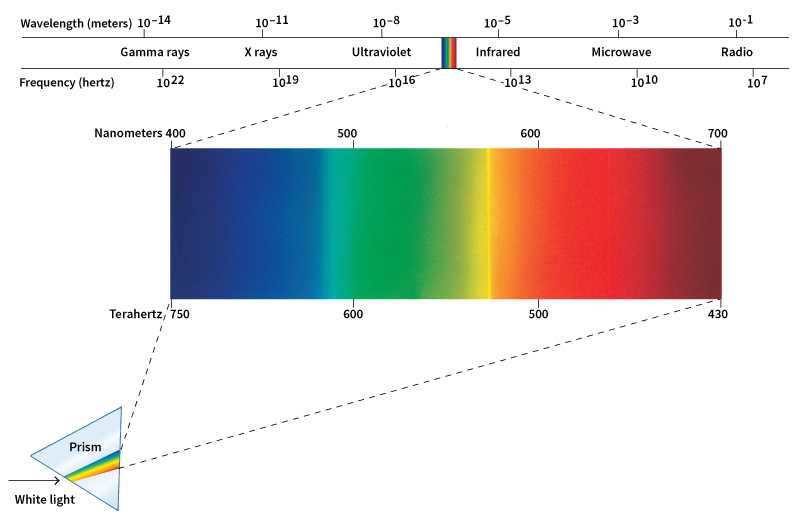
Light waves, like some water waves and waves in a rope, are transverse waves. We can picture some important features of light waves by using the rope as an example. The greatest distance of a crest or trough from the ray or resting level of the rope is called the wave’s amplitude. There are a number of different ways to make wave energy travel down the rope. We can shake the rope up and down to produce a vertical wave, or we can shake it from side to side to make a horizontal wave. The direction of the oscillations is called the wave’s polarization. If, instead of continuously shaking the rope, we give it a short shake, a brief wavelike disturbance called a wave packet will travel down the rope. One way to understand how light can travel as small units of energy is to think of these units as short wave packets.
Electromagnetic waves.
If light is a wave, then what substance oscillates? Scientists of the 1800’s assumed that light waves must travel through some kind of material, just as water waves travel through water. Although they had no evidence of this material, scientists called it the ether. But by the early 1900’s, scientists had concluded that light can travel through empty space. 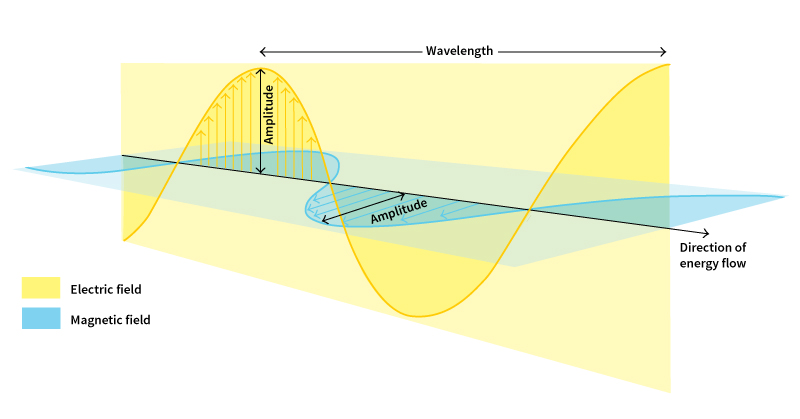
In 1864, the British physicist James Clerk Maxwell found that light is related to electricity and magnetism. According to Maxwell’s theory of electromagnetism, electric fields and magnetic fields together form light waves. These fields consist of electric and magnetic influences acting throughout regions of space with which they are associated. The fields determine the forces that electrically charged objects experience at each point in space and moment in time. When the fields oscillate regularly and travel together through space, they are called electromagnetic waves.
In the electromagnetic wave model, light waves travel along a line much like a ray. The electric and magnetic fields oscillate in directions perpendicular (at right angles) to the ray. We can picture these fields using short arrows that vary in length. The overall pattern of the tips of the arrows for the electric field looks like a wave. Arrows representing the magnetic field also resemble a wave, but these arrows make right angles to the arrows representing the electric field. As the fields oscillate, energy moves along the ray. The amplitude of the waves is the largest value that the fields have as they oscillate.
Just as the direction of oscillations in the rope determines the polarization of the rope wave, the polarization of a light wave depends on the direction of oscillations of the electric and magnetic fields. In most of the light we see, the direction of the oscillations changes rapidly and randomly. Such light is called unpolarized. If the oscillations remain in the same direction, the light is called polarized. When sunlight reflects from a road or from the surface of water, its polarization is often horizontal. You can block such light by wearing sunglasses with polarizing filters. Vertical polarizers will block horizontally polarized light.
Electromagnetic waves include visible light waves, such high-frequency waves as gamma rays and X rays, and such low-frequency—or long-wavelength—waves as radio waves. Maxwell’s theory predicts that all electromagnetic waves travel at only one speed. This speed matches the known speed of light in empty space.
Photons.
In the early 1900’s, scientists confronted some observations about light that could not be explained in terms of electromagnetic waves. In 1900, the German physicist Max Planck proposed an equation to describe the emission of light at the surface of certain types of hot objects called black bodies. Planck could explain the equation only by assuming that the tiny objects emitting light from the surface, now known to be molecules and atoms, emit energy in “chunks” which came to be called quanta. In 1905, the German-born physicist Albert Einstein proposed that light itself consists of quanta that were later called photons. Scientists have measured individual photons and verified that light can indeed behave like a particle. 
In this model, one way to interpret a ray of light is to think of it as the path taken by a photon. Experiments have shown that if a photon has only one possible path from one place to another, then light does behave like a small particle traveling along a ray. However, if the photon can take more than one path, then it behaves like a wave. It takes all paths and so can interfere with itself.
In 1913, the Danish physicist Niels Bohr proposed that the electrons in atoms can have only certain values of energy. Scientists now know that when an atom becomes excited, either by a collision or by light striking it, its electrons can gain only certain amounts of energy. Likewise, when an atom de-excites by emitting light, its electrons can emit only photons with certain energies. 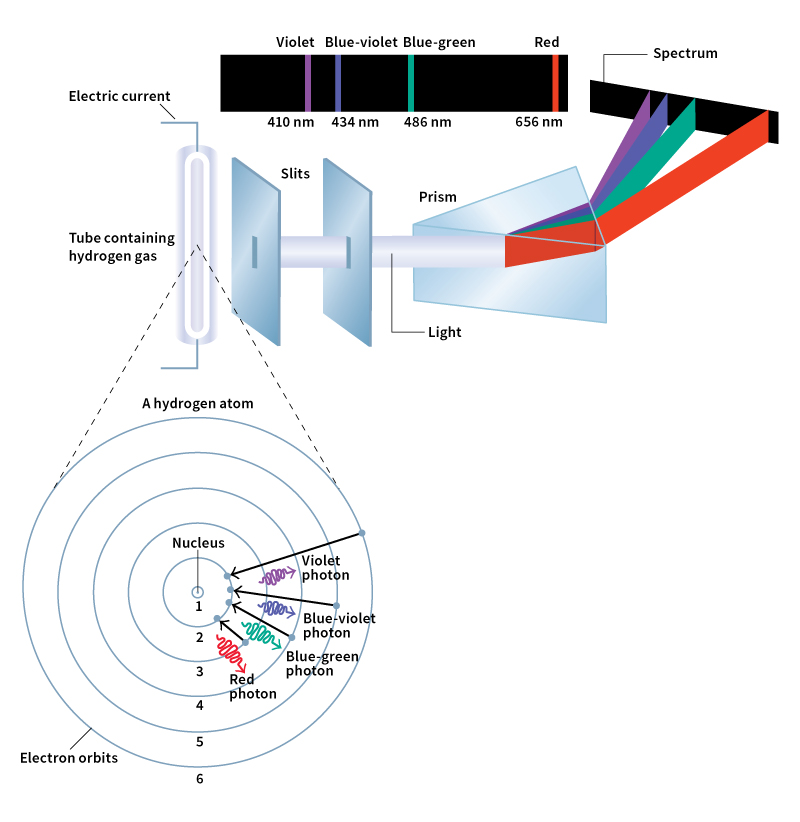
The discoveries of Planck, Einstein, Bohr, and many others concerning light began a revolution in physics known as quantum mechanics. This work provided a new way of describing the strange behavior of extremely small things. In the 1920’s, scientists continued to develop these ideas and test them with experiments. They discovered the astonishing fact that—just as light can behave as a particle—matter, such as electrons, can also behave like waves. See Quantum mechanics.
How light behaves
Scientists study light by observing how it interacts with itself and with matter. They use their knowledge of how light behaves to develop new uses of light in technology. The study of light has led to the invention of such instruments as microscopes and telescopes. It also helps us to understand vision and to answer such fundamental questions as why the daytime sky is blue.
Reflection and absorption.
We can understand some of the behaviors of light by first considering how other types of waves behave. When an incoming water wave strikes a sea wall, it appears to bounce off the wall, becoming an outgoing wave. This bouncing is called reflection. In contrast, when a water wave rolls onto a beach, the beach takes in most of the energy, an effect called absorption.
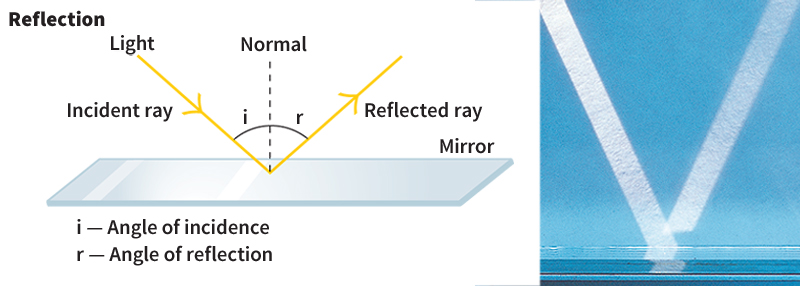
Reflection and absorption also happen to light. When light strikes such shiny metal surfaces as silver, gold, or aluminum, nearly all of the energy is reflected. The small amount of light that is not reflected is absorbed by the metal surface. The reflection of light from such a surface resembles a bounce. Imagine a line perpendicular to the reflecting surface. Such a line is usually called the normal. The angle between the path of an incoming ray and the normal is called the angle of incidence. The reflected ray makes the same angle to the normal as the incoming ray, but on the other side of the normal.
Some materials transmit more light than they reflect or absorb. That is, they allow significant amounts of light to pass through them. When light traveling through the air hits the surface of a smooth pool of water, for example, the pool reflects only some of the light. As a result, you may see a dim reflection of trees and the sky on a calm pond. However, much of the light passes through the surface and continues traveling inside the water.
Refraction.
When a ray passes from air into water, it bends toward the normal at the surface, an effect called refraction. To observe refraction, place a pencil in a glass of water and then look at the pencil from the top on one side. The pencil appears bent at the water surface. The light from the top part of the pencil comes directly to your eyes. The rays from the bottom part pass through the surface between the water and the air. There the rays refract, and so they seem to come from a pencil bottom bent from the pencil top. See Refraction. 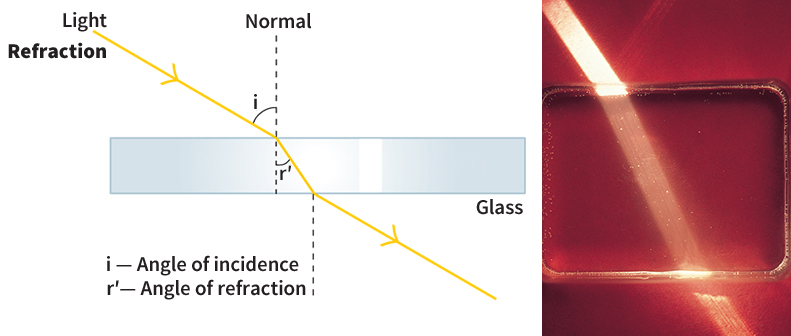
Refraction results from a change in speed as light travels from one material to another. The speed of light in a material is determined by a factor called the refractive index. The greater the refractive index, the slower the light travels in the material. A material’s refractive index depends on many factors, including the material’s density and the types of atoms it contains. Light travels fastest in empty space, which has a refractive index of 1. Water has a refractive index of about 1.33, so the speed of light in water is 1/1.33, or about three-quarters, of its speed in empty space. Some semiconductors, materials that conduct electric current better than insulators like plastic but not as well as conductors like copper, have refractive indices as high as 3.5. In such materials, light travels at less than one-third of its speed in empty space.
Researchers have also made materials with negative refractive indices. When light enters these materials, the refracted ray lies on the same side of the normal as the incoming ray. If a pencil could be dipped in a glass of such a material, it would appear to bend backward.
Light can also be reflected at the junction of two materials with different refractive indices. For example, if you swim underneath a pool’s surface and look up, you will see that the surface of the water appears mirrorlike. This happens because some light rays traveling in a clear material can reflect off the boundary of another material with a lower refractive index. Because air has a lower refractive index than water, some light rays in the pool are reflected and become trapped, a process called total internal reflection. An optical fiber makes use of this effect. It consists of a tiny strand of glass surrounded by a layer with a lower refractive index. Light passing through the glass is confined inside it by total internal reflection. Even if the fiber bends, rays travel down its full length. 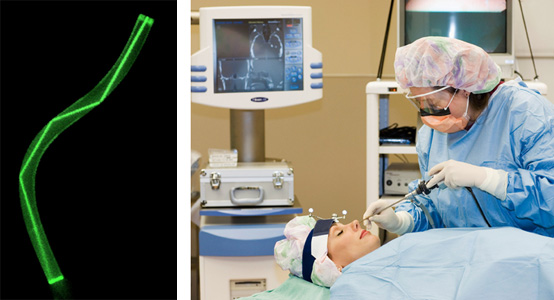
Scattering
occurs when light rays strike atoms, molecules, other tiny particles, or rough surfaces. The particles send the rays of light off in new directions—that is, they cause the rays to scatter. Most of a clear sky appears blue because air molecules scatter more higher-frequency, or bluer, rays of sunlight than they do lower-frequency, or redder, rays. As a result, we see blue light coming from nearly every direction in the sky. The red and orange light scatters less, and so it travels in a relatively straight path. But when the sun is near the horizon, the light reaching us has traveled through more air. As a result, it looks orange or red because it has lost so much of its other colors by scattering.
A transparent material, such as a calm pool of water, lets light pass through it with little scattering. We can see through such a material. A translucent material also allows light to pass through it, but the material scatters each wave in many directions. As a result, we cannot see clearly through such materials as frosted windows. An opaque material blocks all light. You can carry out a simple scattering demonstration by adding just a few drops of milk to a clear glass of water. After only a few drops, the water will become cloudy and translucent. If you add enough milk, the mixture will become opaque. Eventually, it will become white because the milk scatters all wavelengths back toward your eye.
Interference.
When two light waves travel through the same region, they interfere with each other. Certain parts of the waves may be “in phase,” so that the crests of the two waves—and their troughs—overlap and enhance each other. Scientists call this effect constructive interference. Other parts of the waves may be “out of phase,” so that one wave’s crests overlap the other wave’s troughs. The waves cancel each other, a process called destructive interference. When the combined light of the interfering waves strikes a surface, it forms an interference pattern of bright and dark regions called fringes. Regions of constructive interference often appear as bright lines or circles. Areas of destructive interference appear dark. 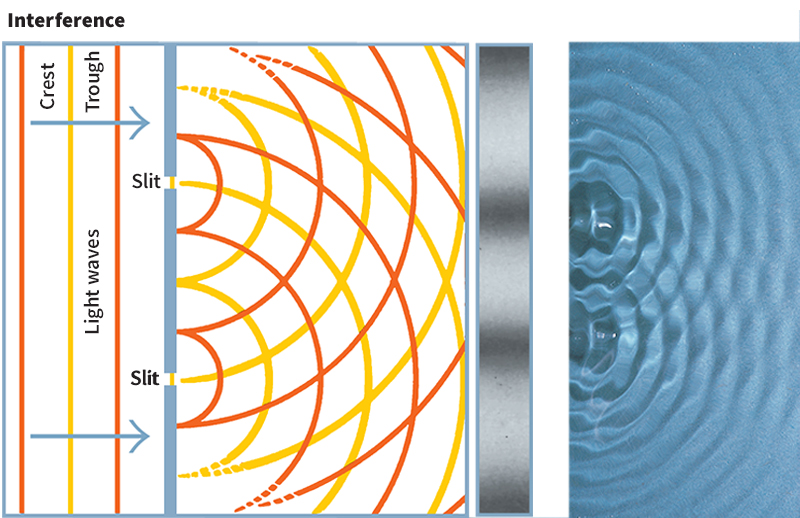
Scientists have demonstrated interference by passing light through two narrow slits and onto a screen. A pattern of bright and dark fringes spreads out over the screen. Some people account for the interference pattern by assuming that photons from one slit interfere with photons from the other slit. However, physicists have shown that an interference pattern will result even if light is passed through the slits one photon at a time. According to quantum mechanics, the photon behaves as a wave, taking both paths and interfering with itself.
Diffraction.
Light that passes through a small opening, such as a pinhole, will spread out in space. This spreading is called diffraction. Light also diffracts when it passes near a sharp edge. For example, careful measurements of the shadow of the sharp end of a razor blade show that light spreads at the shadow’s edge. 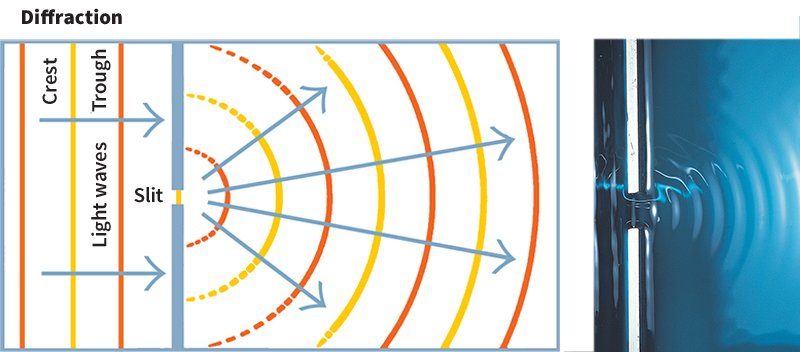
Diffraction can limit our efforts to make a perfect image or to magnify small objects. Imagine, for example, that you attempt to see a tiny object by using a high-quality microscope. As you zoom in, the object’s edges will appear to blur because light diffracts off them.
However, diffraction can also serve a purpose in technology. A device called a diffraction grating can separate the colors in a beam of light for study. The grating consists of thousands of thin slits that diffract light. Each color in the light diffracts by a slightly different amount. The spread of colors can be large enough to make each color visible. A grating used with a telescope can separate the colors in the light from a star, enabling scientists to learn what materials make up the star. A grating used with an optical fiber can combine many wavelengths of light so that the fiber can carry many signals on one small strand of glass.
Dispersion.
In some materials, light with different frequencies, or wavelengths, travels at different speeds. As a result, light spreads into its colors, a process called dispersion. In a prism, for example, white light separates into the colors of the visible spectrum. Diffraction and scattering can also disperse light.
The Doppler effect.
When scientists view the spectra of such rapidly moving sources as distant stars, they find that the light is shifted in frequency. This effect resembles the change in pitch of a train whistle heard by a bystander as the train passes. As the train nears, the listener hears a higher pitch or frequency of sound waves. As it moves away, the listener hears a lower pitch. Likewise, an observer viewing the light waves emitted by a moving object will see them shifted to a different frequency, or color. This change in the frequency of waves caused by the relative motion of the wave source and its observer is called the Doppler effect. See Redshift (The Doppler redshift).
Light and measurement
Because light behaves like a wave that oscillates in time, physicists often describe light in terms of its frequency. One common unit, the hertz, equals the number of oscillations per second. The frequency of visible light measures nearly one thousand trillion hertz. This means that one thousand trillion crests and troughs of a given light wave enter your eye each second. However, the wavelength of visible light measures only about 20-millionths of an inch, or half a millionth of a meter.
The oscillations of a light wave can be finer and more regular than the markings on any ruler. Therefore, scientists often use lasers, which have single, stable frequencies, to make precise measurements. Such devices can measure, among other things, the speed, wavelength, and frequency of light. Scientists use the interference of laser beams to measure extremely small distances. Devices called interferometers can measure distances to an accuracy of one nanometer, smaller than the diameter of many molecules.
The speed of light.
Light seems to travel across a room the instant a light bulb is turned on. But while light does travel quickly, its speed is not infinite. The speed of light in empty space is about 186,282 miles (299,792 kilometers) per second. Physicists call this speed invariant because it does not depend on the motion of the light’s source. For example, light that is emitted by a moving flashlight has the same speed as light that is emitted by a stationary flashlight. Einstein took this puzzling fact as a fundamental principle of his theory of relativity [see Relativity (Special relativity)]. Relativity predicts that no object or unit of information can travel faster than the speed of light.
From ancient times, people argued about whether the speed of light is finite (limited) or infinite. During the early 1600’s, the Italian physicist Galileo attempted to settle the argument by measuring the speed of light. He signaled with a lantern to an assistant at a distant hill, hoping to measure the delay in the arrival of the assistant’s response lantern signal. However, light travels too fast for such a measurement, and so Galileo’s effort failed.
About 1675, the Danish astronomer Olaus Roemer proved that light travels at a finite speed. While working in Paris, Roemer observed that the intervals between the appearances of some of Jupiter’s moons varied with the changing distance between Jupiter and Earth. Roemer suggested that these differing intervals occurred because light travels at a finite speed and so takes longer to reach Earth when Jupiter is farther away. He estimated the speed of light at 226,000 kilometers per second, about 25 percent less than modern measurements.
In 1926, the American physicist Albert A. Michelson made one of the first precise measurements of the speed of light. He used a rapidly rotating eight-sided mirror to direct a beam of light to a distant reflector. The returning beam would then reflect to an observer if the rotating mirror was at the correct angle. Michelson adjusted the speed of the mirror until it turned to the correct angle during the time the light traveled to the reflector and back. The speed of the mirror indicated the speed of light to be 299,796 kilometers per second, an error of less than 4 kilometers per second. 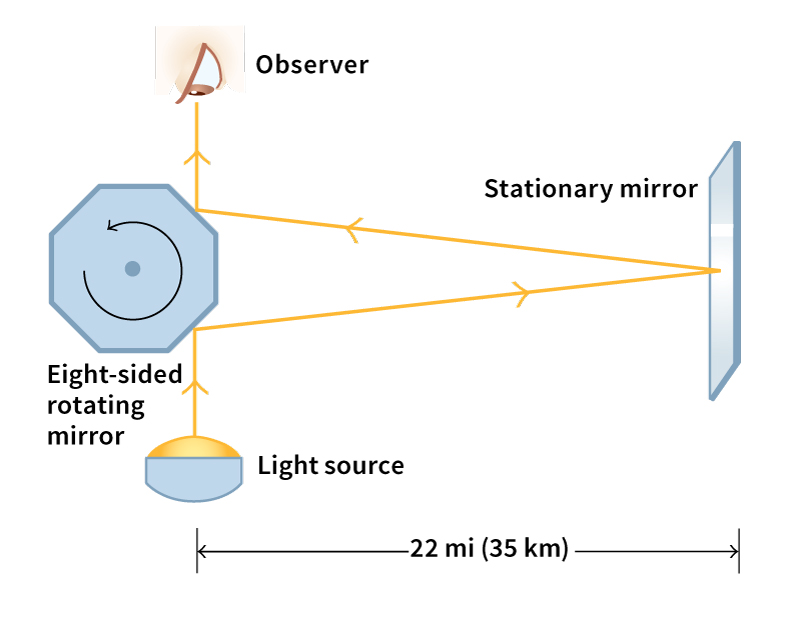
Scientists have measured the speed of light with such accuracy that, in 1983, the 17th General Conference on Weights and Measures defined the meter in terms of the speed of light. One meter equals the distance that light travels in empty space in exactly 1/299,792,458 of a second.

In the 1990’s, physicists used a complex system of optics to slow light in matter to speeds of only several meters per second. In the 2000’s, the scientists stopped and started light by storing it in atoms and then releasing it.
Energy and brightness.
Scientists and engineers use several units to measure the energy and brightness of light. In the International System of Units (SI), the energy of a beam of light is measured in joules. The SI unit for power—the rate of energy flow, or energy divided by time—is the watt. One watt equals 1 joule per second. For example, a 60-watt light bulb emits 60 joules of light energy each second.
The energy from a light bulb spreads out in space so that the power striking a given area decreases with distance. The amount of power per unit area, called the irradiance, is measured in watts per square meter. If you measure the irradiance of a light source from one distance and then move twice as far away, you will find that the irradiance has dropped to one-quarter of its first value. That is, the irradiance varies inversely (oppositely) with the square of the distance from the source. This relationship is called the inverse square law.
Because irradiance varies with distance, scientists use other measures to describe the amount of light given off by a source. For such small sources as light bulbs, they use a measure called radiant intensity. The radiant intensity represents the power emitted into a solid angle, or wedge, of space. It is measured in watts per steradian. A steradian is a standard unit of solid angle. For such broad-area light sources as high-definition television or computer screens, the light is described by the radiance of the source, measured in watts per steradian per square meter.
Engineers often measure visible light sources according to how bright they would appear to the human eye. The portion of radiant intensity detectable by the human eye is called the luminous intensity. Its standard unit is the candela. This name comes from the fact that the light from a standard candle originally defined the unit. The portion of power detectable by the human eye is known as the luminous flux and is measured in lumens. One watt of green light has a luminous flux of about 680 lumens. One watt of deep blue or deep red light has a smaller luminous flux because the eye is less sensitive to blue and red than it is to green light.
Light in technology and industry
Since the early to middle 1900’s, advances in the understanding of light and the development of the electronics industry have enabled scientists to create several important devices based on light. These instruments have transformed such industries as manufacturing and medicine and changed many aspects of our daily lives.
Lasers
rank as one of the most important and useful inventions based on light. Ordinary light bulbs give off light that consists of many colors and that spreads out in many directions. In contrast, lasers emit light with only one color, or wavelength, and the light undergoes little spreading. Light with these qualities is said to be coherent. Such light can travel great distances with little loss of intensity.
The unique characteristics of laser light make lasers useful in many everyday devices. Lasers used in grocery store bar code scanners, for example, can reflect light off a bar code from a relatively long distance. A light detector then measures the pattern of reflected light, which is unique to each item in the store. The scanner then sends the signal to a computer, which identifies the item. Lasers also find use in compact disc players, telecommunications devices, and high-quality printers.
Different wavelengths of laser light are useful for different applications. For example, a surgeon can perform corrective eye surgery with one wavelength of laser light and can safely examine human tissue using a different wavelength. 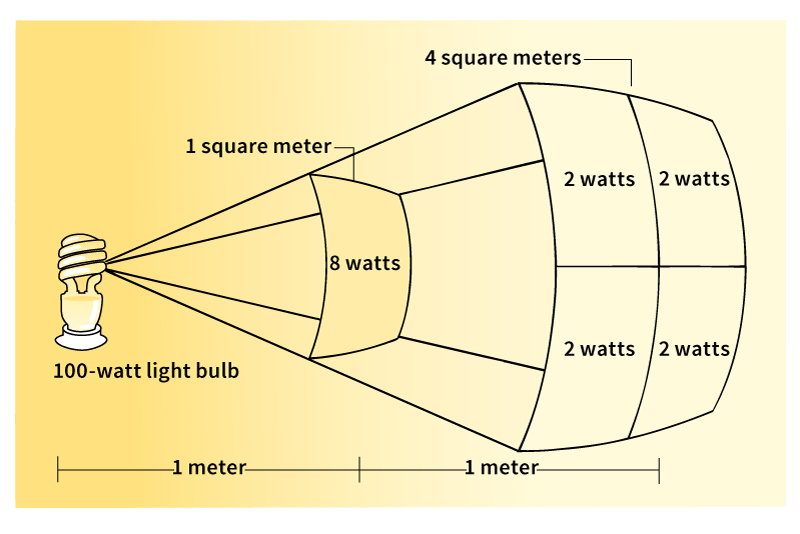
Scientists also use lasers for research on microscopic processes. Devices known as ultrafast lasers can produce pulses of light that last for only a few femtoseconds. One femtosecond is one-millionth of one-billionth of a second. Pulses from these lasers are so brief that they can be used to create images of electrons in mid-flight. Ultrafast lasers have also enabled scientists to view chemical reactions as they occur.
Photonics
involves using light instead of electrons to transmit information. Optical fibers serve as the “wires” for photonic signals in telecommunications. Because many different wavelengths of light can travel down the same fiber without interfering with each other, optical fibers can transmit more data than can metal wires of the same diameter.
Fiber optics also find use in medicine. For example, surgeons can insert optical fibers through tiny incisions to look and work inside the human body. This technique has reduced the cutting required for many procedures.
Photochemistry
is the study of the chemical effects of light. The energy of light can chemically change the surfaces of materials absorbing it. For example, strong light can fade colored fabrics by chemically changing their dyes. Light also changes the chemistry of the eye’s retina, producing the signals involved in sight. By exciting electrons in molecules, light can begin and control chains of chemical reactions. Such a chain of reactions makes photosynthesis possible.
Photoelectric effect.
When certain materials absorb light, the light’s energy frees electrons from atoms on the materials’ surface. Scientists call this a photoelectric effect. In some devices, these freed electrons can flow through a circuit as electric current. In other devices, light can increase a circuit’s ability to conduct current from a battery. Digital cameras and electric eyes use photoelectric effects to measure changes in light.
One photoelectric effect, called the photovoltaic effect, involves semiconductors. Some of these materials have extra electrons, and some have shortages of electrons in the form of positively charged “holes.” When both types of semiconductors are pressed together, light falling on the junction between them causes a flow of electrons and holes that can produce electric power. This effect enables solar cells to change sunlight into electric energy. In LED’s, by contrast, an electron can move into a hole, emitting its extra energy as light.
Light and energy efficiency.
The light-based inventions of the middle and late 1900’s have led to novel devices that use light to generate power and help conserve energy. Solar cells, for example, provide electric power for spacecraft, emergency telephones along highways, and handheld calculators and watches.
In the early 2000’s, LED’s began to replace many conventional light bulbs. Some LED’s last 100 times longer than the best incandescent bulbs and 10 times as long as the best fluorescent bulbs. For these reasons, manufacturers now use LED’s to make traffic signals. Additional uses of LED’s include remote controls, brake lights, and backlights for some flat-panel televisions.
Energy-efficient windows called low-emissivity windows use principles of optics to reduce the transfer of heat through the window glass. The glass has a coating that transmits visible light but reflects longer-wavelength heat energy, keeping heat in during the winter and out during the summer. As a result, such windows reduce energy costs from heating and air conditioning.
Light in manufacturing.
Engineers have developed ways to use light to improve manufacturing methods. By combining cameras and other light detectors with computers, they have developed machine vision systems. These devices can produce three-dimensional images of manufactured objects and even guide robotic tools with high accuracy and speed. Machine vision systems can also inspect finished products ranging from computer chips to automobiles. Because they use light, these systems can examine sensitive products without contaminating them through surface contact.
Scientists have also found ways of using light to create new products. For example, electronics manufacturers use a technique called photolithography to make printed circuit boards and the tiny circuits used in computer chips. The process employs light to imprint electronic pathways onto light-sensitive chemicals called photoresists.
Light in medicine.
Doctors use a variety of optical instruments to examine the body and to identify and treat medical conditions. For example, many hospitals measure the oxygen level in a patient’s blood using a small, painless device that illuminates the finger. The light is absorbed by hemoglobin, the pigment in red blood cells that carries oxygen. A detector measures the oxygen level by calculating the difference in absorption between oxygen-rich and oxygen-poor hemoglobin. A doctor then decides if the patient needs more oxygen.
Doctors may also detect health problems by placing fluorescent molecules called fluorophores in a patient’s body. Fluorophores absorb light at one wavelength and emit longer-wavelength light in response. For example, a fluorophore called fluorescein absorbs blue light and emits yellow-green light. Doctors use fluorescein to diagnose certain eye diseases. They first inject fluorescein into a patient’s bloodstream, which carries it to the eye. Doctors then shine blue light inside the eye and use the yellow-green light emitted by the fluorescein to record images of blood vessels. These images can help diagnose such circulation problems as leaky blood vessels.
Different types of microscopes use light to form images of cells and tissues, helping doctors diagnose medical conditions. Doctors use confocal microscopes to produce three-dimensional images of a specimen. These devices typically shine laser light on different layers of a sample containing fluorescent material and then record the emitted light to produce an image with depth. Doctors often use confocal microscopes to examine human tissue and identify cancer cells.
Light is also used in medicine to correct a person’s eyesight. Using a laser, a doctor can reshape the eye’s cornea, or outer coating, changing the way it focuses light and so improving vision. Eye doctors can also use a technique called adaptive optics to view individual blood cells and light receptors in the retina. Light bounced off the retina typically undergoes small distortions as it passes out through the cornea and other parts of the eye. Adaptive optics uses mirrors whose shape can be altered to compensate for these distortions.
Studying light.
For many years, students wishing to study light did so by first earning college degrees in such related fields as physics and mathematics. During the 1800’s, scientists in many parts of the world developed technology that enabled them to design optical instruments and make precise measurements with light. But by the early 1900’s, Germany had become the center of optics studies. After World War I (1914-1918) began, schools devoted to the study of light were also established in Paris and in London. Today, academic institutions around the world award degrees in optics.
In the United States, leaders of the photographic equipment manufacturer Eastman Kodak and the optics company Bausch & Lomb called for a school specializing in the study of light. In 1929, they helped found the Institute of Applied Optics (now the Institute of Optics) at the University of Rochester in New York. In 1964, the University of Arizona established the Optical Sciences Center (now the College of Optical Sciences) in Tucson. Such professional societies as the Australian Optical Society, the Optical Society of America, the European Optical Society, and SPIE (also known as the Society of Photo-Optical Instrumentation Engineers) actively promote the study of light.
