Lithium << LIHTH ee uhm >> is a soft, silvery-white metallic element, the lightest known metal. Lithium is only half as heavy as an equal volume of water. It reacts with water, as does sodium, to release hydrogen gas. But, unlike sodium, the reaction usually does not ignite the hydrogen.
Lithium is a key component of some batteries. Lithium batteries store energy efficiently and last longer than other common kinds of batteries. Electric cars, electric scooters, laptop computers, cell phones, power tools, and electronic cigarettes often use lithium batteries.
Lithium compounds are used in the manufacture of various materials, including ceramic products, enamels, glass, and lubricants for use at high temperatures. They are also used in rubber products and in dyes for textiles. One compound, lithium carbonate, is used as a drug to treat bipolar disorder.
Most of the world’s lithium output comes from Australia. Chile and China are also major lithium producers.
Lithium metal does not occur in nature because it combines easily with other elements. Chemists obtain the metal by passing electric current through fused (melted) lithium chloride.
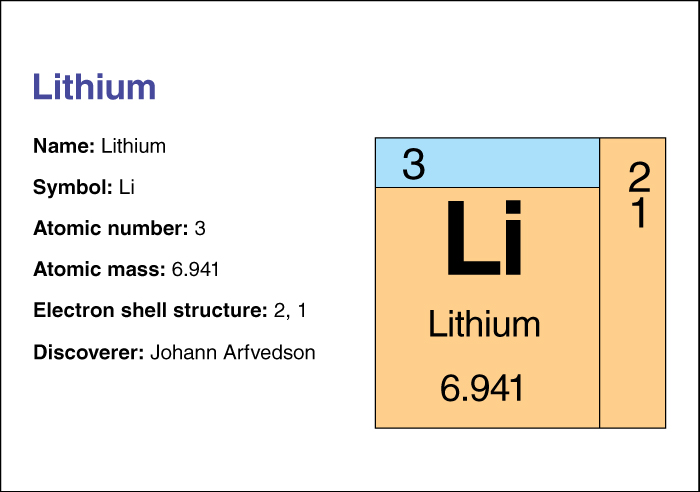
The chemical symbol for lithium is Li. Its atomic number (number of protons in its nucleus) is 3. Its relative atomic mass is 6.941. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Lithium melts at 180.54 °C and boils at 1347 °C. It was discovered in 1817 by Johann August Arfvedson, a Swedish chemist.
Chemists classify lithium as an alkali metal. For information on the position of lithium on the periodic table, see the article Periodic table.
