Lutetium, << loo TEE shee uhm >> (chemical symbol, Lu), is one of the lanthanide metals. It has an atomic number (number of protons in its nucleus) of 71. Its relative atomic mass is 174.967. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. For information on the position of lutetium on the periodic table, see the article Periodic table .
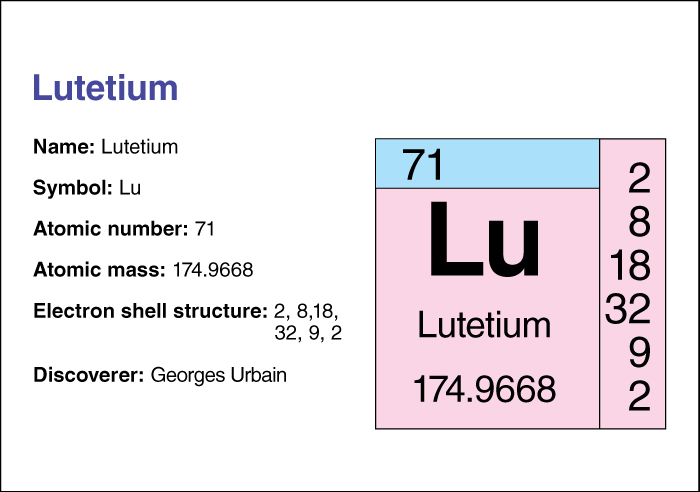
The name lutetium comes from Lutetia, the ancient name for Paris. The French scientist Georges Urbain discovered lutetium in 1907. He developed a process for separating the original element ytterbium into two elements, lutetium and ytterbium, or neoytterbium. Lutetium is found with ytterbium in the minerals gadolinite, xenotime, and other minerals that contain lanthanides. The metal has a silver color. It has a melting point of 1663 °C and a boiling point of 3402 °C. It has a density of 9.84 grams per cubic centimeter at 25 °C (see Density ), making it the densest lanthanide element.
See also Rare earth ; Ytterbium .
