Metal is any of a large group of chemical elements that includes copper, gold, iron, lead, silver, tin, and other elements that share similar qualities. Nearly 80 percent of the known elements are metals. Metals make up much of Earth. They are important in all aspects of construction and manufacturing. Manufacturers use metals to make tools, utensils, jewelry, and a wide variety of machinery. Compounds that contain metals are used in drugs, batteries, and many other products.
What metals are.
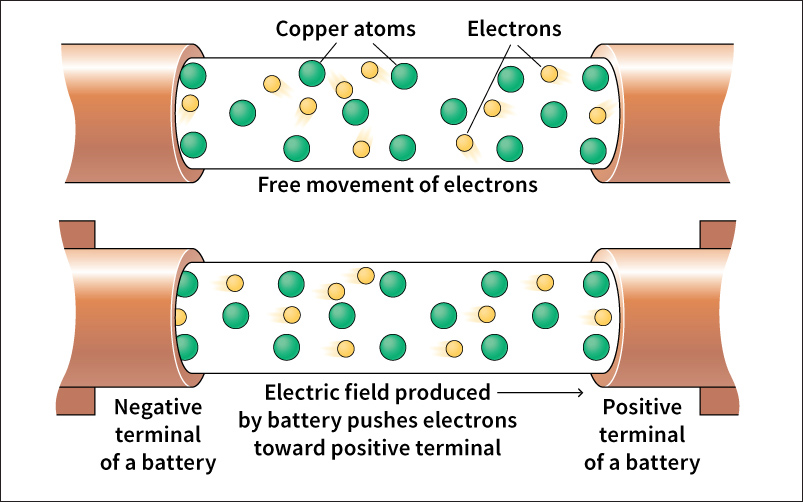
Several properties (characteristics) distinguish metals from other materials. Metals often appear shiny because they reflect light well. They typically serve as good conductors of heat and electric current. Metals can be shaped into useful objects because they are malleable (able to be hammered into thin sheets) and ductile (able to be drawn out into wires). Alloys are combinations of metals that retain metallic properties. Common alloys include bronze, brass, and gun metal.
Metals often form chemical compounds whose properties differ from their own properties. For example, table salt (sodium chloride) is a colorless, brittle solid formed from the reaction of sodium, a soft, silvery metal, with chlorine gas. Rust consists of iron oxides that form when iron or steel reacts with the oxygen in air.
Metals often form compounds with nonmetals by transferring electrons to the nonmetal. Metals that lose electrons in this way are said to be oxidized. Some metals oxidize more easily than others do. Metals that are difficult to oxidize often occur in nature in native (relatively pure) deposits. Metals that oxidize easily usually occur in chemical compounds. The precious metals gold, silver, and platinum are difficult to oxidize and appear as native deposits. Copper, which is more easily oxidized, can occur in native deposits but more often appears in compounds, such as turquoise and azurite. Aluminum, iron, magnesium, potassium, sodium, and zinc are so easily oxidized that they never occur as native deposits in Earth’s crust.
Where metals are found.
Geologists think Earth’s core consists mainly of pure molten iron. Nearly all of the metal in Earth’s crust, however, occurs in compounds. Aluminum makes up about 8 percent of the crust, iron about 5 percent, and calcium 4 percent. These metals mainly occur in compounds with carbon, oxygen, phosphorus, silica, and sulfur. Common compounds include the minerals alumina (Al2O3), magnetite (Fe3O4), hematite (Fe2O3), and calcite (CaCO3).
The universe contains relatively small amounts of metal. Together, the nonmetallic elements hydrogen and helium make up more than 99.9 percent of all visible matter. Of the rest, the most abundant metals are, in order, magnesium, iron, aluminum, calcium, sodium, nickel, chromium, manganese, potassium, titanium, cobalt, zinc, vanadium, and copper. On Earth, hydrogen occurs as a gas. But under high pressure, hydrogen can behave like a metal. Many scientists believe that the planet Jupiter is composed mostly of hot, metallic liquid hydrogen under extreme pressure.
Metals through the ages.
Ancient people used native copper, gold, and silver to make ornaments, plates, jewelry, and utensils. Production of copper and tin alloys led to the Bronze Age, when bronze replaced stone as the chief toolmaking material. In some areas, such as the Near East, the Bronze Age began about 3000 B.C. The Iron Age followed around 1,000 B.C., when iron became the chief toolmaking material. Iron and steel are still widely used in construction and manufactured products today. Aluminum became an important metal after chemists developed techniques for separating it from other elements in the 1800’s. In the 1900’s, engineers developed electric generators and weapons that used uranium and other radioactive metals as fuel.
