Molybdenum, << muh LIHB duh nuhm, >> (chemical symbol, Mo) is a hard, silvery-white metallic element. It melts at a higher temperature than most other metals, about 2623 °C. Manufacturers add molybdenum to steel and to nickel to make strong, heat-resistant alloys (combinations of two or more metals). Molybdenum metal is widely used in aircraft and missile parts. Molybdenum sulfide (MoS2) serves as a high-temperature lubricant. It also catalyzes (stimulates) chemical reactions that remove unwanted sulfur from petroleum.
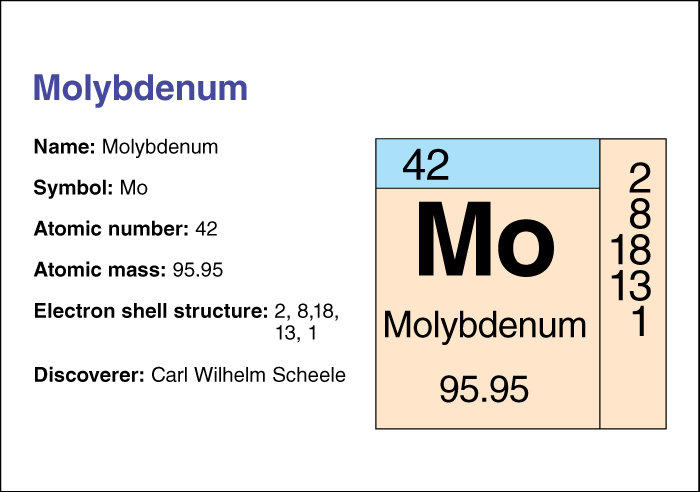
Molybdenum’s name comes from the Greek word molybdos, meaning lead, because people once mistook it for lead. In 1778, the Swedish chemist Carl Wilhelm Scheele determined that molybdenum was a separate element. Molybdenum occurs in nature in an ore called molybdenite, made up of molybdenum sulfide, and in several other minerals. China, Chile, and the United States produce most of the world’s molybdenum. A large unmined deposit near Ketchikan, Alaska, contains more than 10 percent of the world’s known reserves of molybdenite.
Molybdenum’s atomic number (number of protons in its nucleus) is 42. Its relative atomic mass is 95.94. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most stable isotope of carbon. Molybdenum boils at around 4639 °C. Chemists classify molybdenum as a transition metal . For information on the position of molybdenum on the periodic table, see the article Periodic table .
