Neodymium, << `nee` uh DIHM ee uhm >> (chemical symbol, Nd), is a metallic element belonging to the lanthanide group. Its atomic number (number of protons in its nucleus) is 60. Its relative atomic mass is 144.24. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. For information on the position of neodymium on the periodic table, see the article Periodic table .
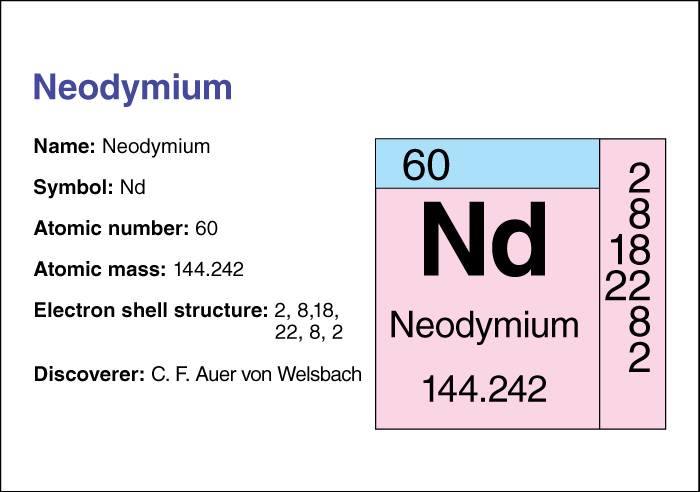
C. F. Auer von Welsbach of Austria discovered neodymium in 1885. He separated the so-called element didymium into neodymium and praseodymium.
Neodymium melts at 1021 °C, and boils at 3074 °C. It has a density of 7.003 grams per cubic centimeter at 25 °C. The metal can be prepared by electrolysis of its halide salts, or by the reduction of these salts by alkaline earth metals in the presence of heat. The ceramic industry uses salts of neodymium to color glass and in glazes. The metal is present in misch metal, an alloy with many uses, and in the important Nd-YAG laser.
