Neutron is a subatomic particle. Neutrons, together with subatomic particles called protons, form the nuclei of all atoms except ordinary hydrogen, whose nucleus consists of a single proton. Neutrons and protons make up 99.9 percent of an atom’s mass. A cloud of electrons around the nucleus accounts for the rest of the mass. In the nucleus, neutrons and protons are held together by a force known as the strong interaction or the strong nuclear force.
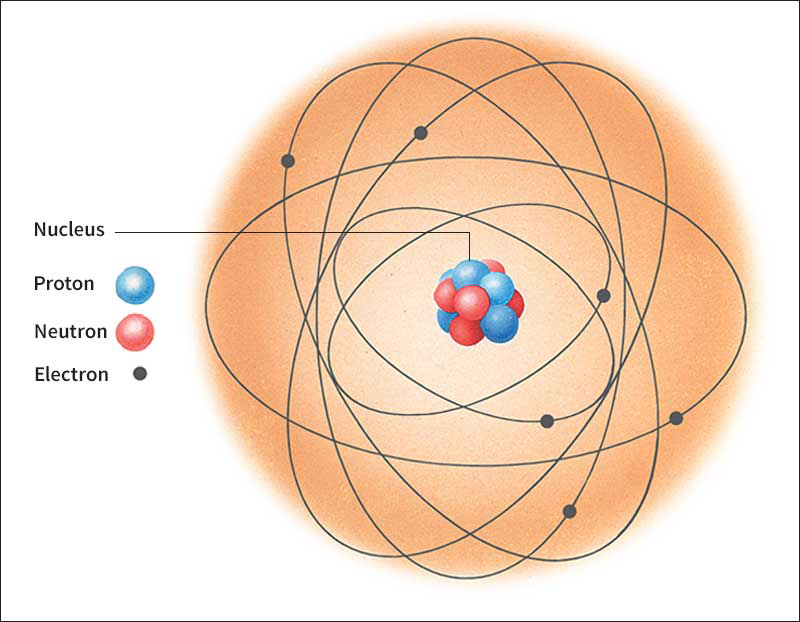
The number of neutrons in an atom of any chemical element is equal to the difference between the element’s mass number (total number of protons and neutrons) and its atomic number (number of protons). The atoms of lighter elements contain about an equal number of neutrons and protons. Heavier elements contain more neutrons than protons.
Neutrons consist of fundamental particles called quarks. A neutron has no electric charge. Its diameter is approximately one millionth of a nanometer. One nanometer equals one millionth of a millimeter, or 1/25,400,000 inch. The mass of a neutron is slightly greater than that of a proton. A free neutron decays into a proton, an electron, and an antineutrino. Free neutrons have an average life span of about 15 minutes.
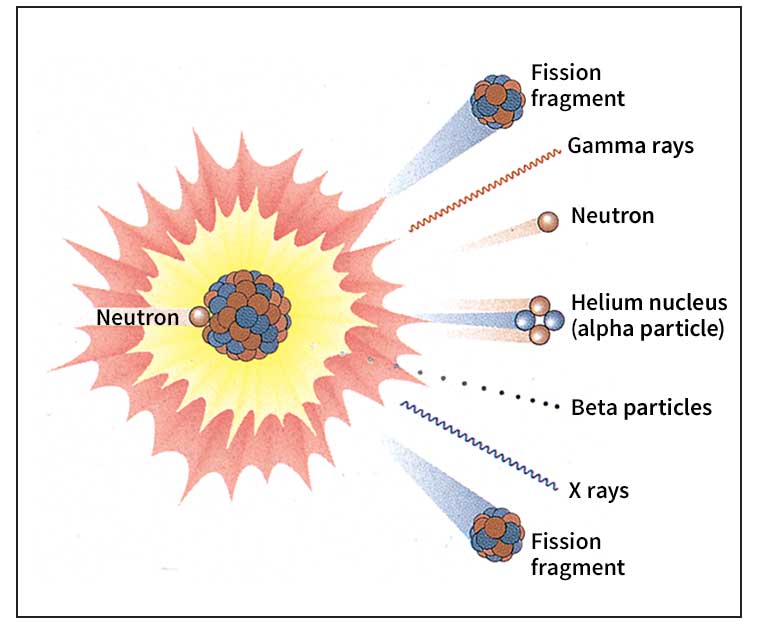
Sir James Chadwick, a British physicist, discovered the neutron in 1932. Today, scientists use neutrons to make various elements radioactive. They bombard atoms of the elements with neutrons in a nuclear reactor. After the nuclei of the atoms absorb neutrons, they decay by giving off radiation. When a nucleus of the uranium isotope U-235 is struck by a neutron, it becomes unstable and splits into two nearly equal parts. This process, called fission, releases a huge amount of energy and frees additional neutrons that cause more uranium nuclei to fission. A continuous series of such fissions, called a nuclear chain reaction, produces the energy in nuclear weapons and nuclear reactors.
