Palladium << puh LAY dee uhm >> is a soft, shiny, silvery-white metal. It is one of six platinum metals, and is relatively rare. Palladium is often used in place of platinum because it is cheaper, harder, and lighter than platinum. It is found with deposits of other platinum metals, with nickel-copper ores, and with mercury.
Palladium can be drawn into wire or hammered into sheets. It is often mixed with gold to make “white gold” jewelry. Palladium is also used in making surgical instruments.
Element, Chemical (table: Table of the elements)
Finely divided palladium, called palladium black, is used as a catalyst, a substance that causes or speeds up chemical changes. It is an important catalyst in a process called hydrogenation, which is used to improve the quality of oils and to prepare gasoline. Automakers use palladium, together with platinum and rhodium, in catalytic converters. These devices reduce the pollutants given off by auto engines (see Catalytic converter ).
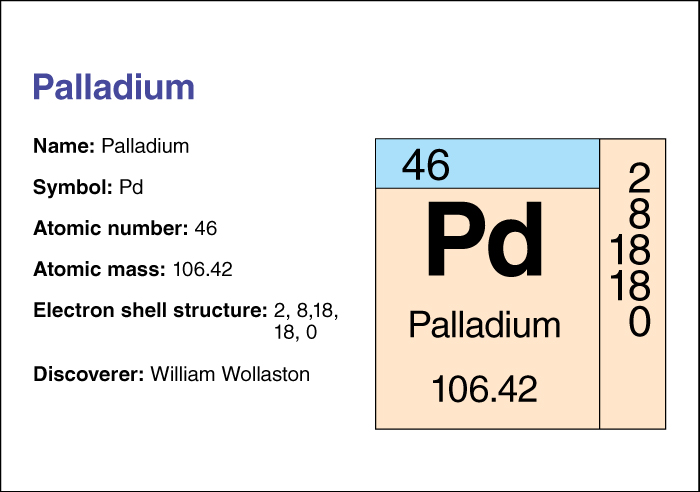
The English chemist William H. Wollaston discovered palladium in 1803. It has the chemical symbol Pd. Its atomic number (number of protons in its nucleus) is 46. Its relative atomic mass is 106.42. A chemical element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Palladium melts at 1552 °C and boils at 2940 °C. It has a density of 11.99 grams per cubic centimeter at 20 °C. Palladium forms simple compounds with elements such as hydrogen, oxygen, and chlorine. Chemists classify palladium as a transition metal . For information on the position of palladium on the periodic table, see the article Periodic table .
