Polypropylene, << `pol` ee PROH puh leen, >> is a strong, lightweight, and heat-resistant plastics material. The material is durable but can be molded into a thin object that bends without breaking. Products made of poly-propylene include panels for automobile interiors, coatings on telephone wires, carpet fibers, ski apparel, and containers for such food products as yogurt and margarine.
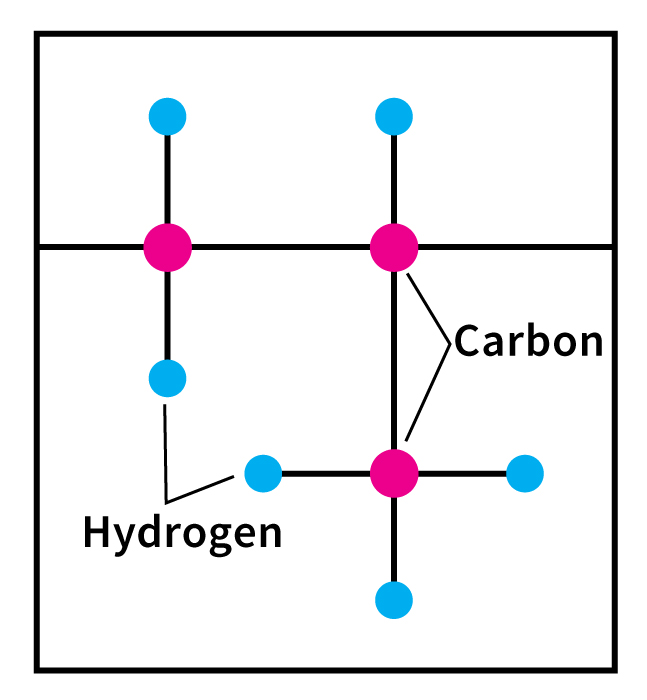
Polypropylene is a synthetic polymer. A polymer is a long, chainlike molecule. The “links” are repeating patterns of simple groups of atoms called monomers. Polypropylene is made from propylene monomers, each consisting of three carbon atoms and six hydrogen atoms. The chemical formula of polypropylene is (C3H6)n, where n is the number of monomers. Polypropylene is a thermoplastic–that is, it softens and melts at high temperatures (see Plastics (Types of plastics) ).
Manufacturers make polypropylene by mixing propylene gas with a catalyst, a substance that speeds up a chemical reaction without being used up by the reaction. They can add pigments to change the color of the material and fillers to make it more difficult to burn or to increase its stiffness. Methods used to shape polypropylene products include extrusion (pushing melted plastic through an opening), injection molding (forcing melted plastic into a mold), and thermoforming (using a vacuum to draw hot plastic sheets onto a form).
Giulio Natta, an Italian chemist, developed polypropylene in 1954. He shared the 1963 Nobel Prize for Chemistry with German chemist Karl Ziegler for their work with plastics.
