Protactinium, << proh tak TIHN ee uhm, >> is a radioactive metal belonging to the actinide series of elements . Two teams of scientists independently isolated the element in 1917. These were Otto Hahn and Lise Meitner of Germany, and Frederick Soddy and John Cranston of the United Kingdom. Protactinium occurs naturally in all uranium ores. It is also produced artificially in nuclear reactors and in particle accelerators.
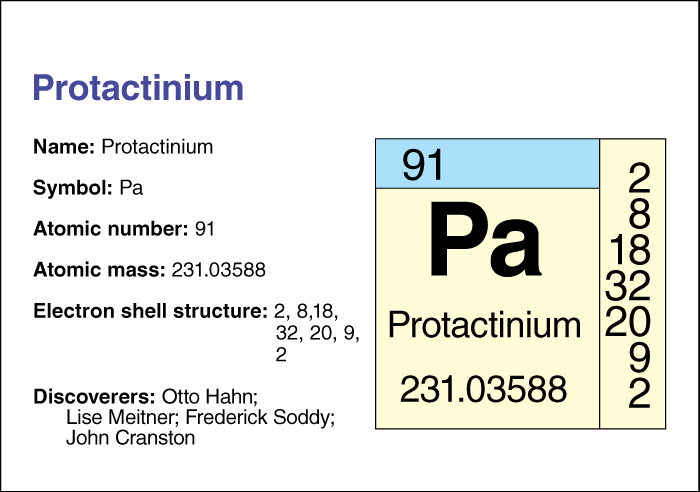
Protactinium has the symbol Pa. Its atomic number (number of protons in its nucleus) is 91. The most stable isotope of protactinium has an atomic mass number (total number of protons and neutrons) of 231. An element’s isotopes have the same number of protons but different numbers of neutrons. Isotope 231 has a half-life of 32,500 years—that is, due to radioactive decay, only half the atoms in a sample of isotope 231 would still be atoms of that isotope after 32,500 years.
For information on the position of protactinium on the periodic table, see the article Periodic table .
The relative atomic mass of protactinium is 231.03588. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant isotope of carbon. Several chemical compounds containing protactinium are known. Protactinium metal melts at 1572 °C.
