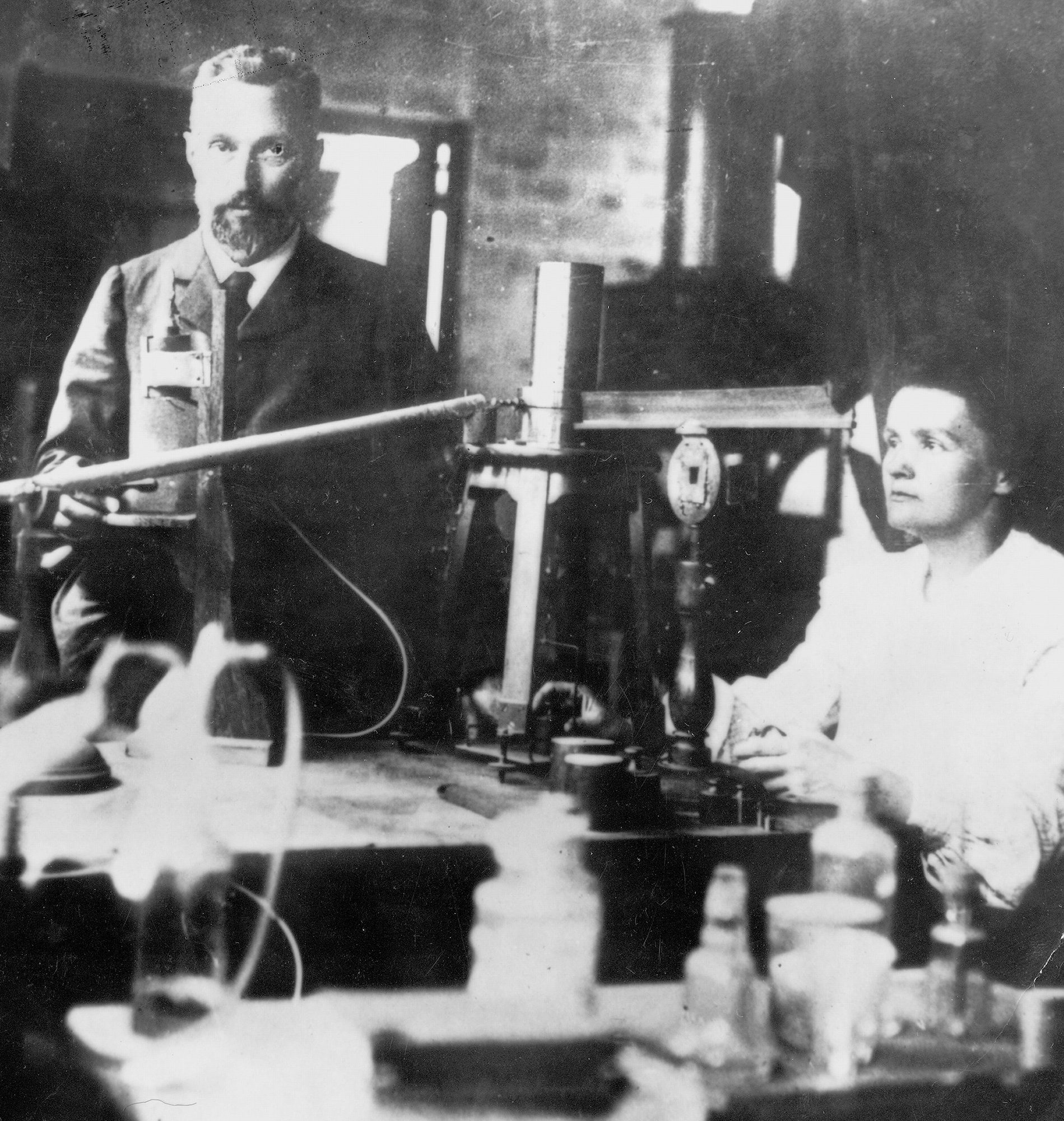
Radium, << RAY dee uhm, >> is a radioactive metallic element found mainly in uranium and thorium ores. The French chemists Marie and Pierre Curie and Gustave Bemont discovered radium in 1898 while working with pitchblende, a uranium ore. They noted that the ore emitted (gave off) more radiation than could be produced by uranium alone. Their work led to the discovery that atoms of one element can transform into atoms of another element through a process called transmutation.
In the past, people used radium in a wide range of applications. Physicians used it in treating cancer because the radiation it emits can kill cancer cells. Today, there are many safer and cheaper ways to produce radiation for medical and other uses.
Radium atoms going through transmutation emit energetic alpha particles, tiny particles made up of two protons and two neutrons. These particles can damage the molecules in living tissue, including the genetic material DNA (deoxyribonucleic acid). Alpha particles cannot travel far through tissue, so radium seldom poses a serious health risk unless it accumulates in the body. In the early 1900’s, manufacturers used radium in fluorescent paint applied to watch and instrument dials. This practice eventually stopped because many workers who applied the paint developed bone cancer. The painters often used their lips to straighten the bristles of radium-contaminated paintbrushes and thus developed harmful accumulations of the radioactive material.
Radium behaves chemically like calcium and tends to travel to the parts of the body where calcium is found—the bones. There, radiation from radium can kill the cells that produce red blood cells or can produce cancers in the bone marrow. Dangerous exposure to radium is rare because little radium persists in the environment.
Loading the player...Atomic transmutation
Properties.
Pure radium is silver-white. Its chemical symbol is Ra, and its atomic number (number of protons in its nucleus) is 88. Chemists classify radium as an alkaline earth metal. For information on the position of radium on the periodic table, see the article Periodic table. Radium has 33 known isotopes. Different isotopes of an element have the same number of protons but different numbers of neutrons. The most common and stable isotope of radium is called radium 226 because it has an atomic mass number (total number of protons and neutrons) of 226. Radium 226 has a half-life of 1,599 years—that is, because of radioactive decay, only half the atoms in a sample of radium 226 will still be atoms of that isotope after 1,599 years. Radium melts at 700 °C and boils at 1140 °C. It has a density of 5 grams per cubic centimeter at 20 °C.
How radium forms and breaks down.
Radium 226 forms during the radioactive decay of uranium 238. The uranium atom emits two alpha particles, eventually becoming an atom of thorium 230. That atom then emits an alpha particle to become an atom of radium 226. Radium 226 decays by emitting an alpha particle, producing the radioactive gas radon 222.
