Rhenium, << REE nee uhm, >> is a rare, costly, silvery-white metal. It is found in small amounts in such minerals as gadolinite and molybdenite. Rhenium has one of the highest melting points of the chemical elements. Because it withstands high temperatures, rhenium is a valuable ingredient in certain alloys (mixtures of metals). It is sometimes mixed with tungsten or platinum to make heat-resistant electrical equipment. It is also used in making filaments (fine wires) for instruments called mass spectrometers that measure the mass of atoms and molecules.
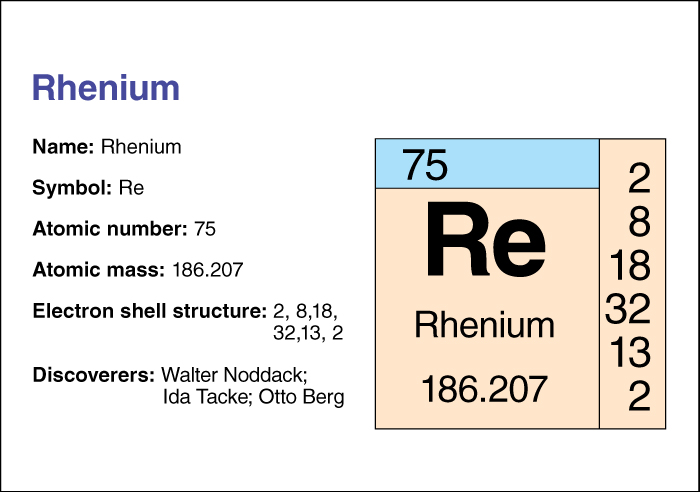
It has the chemical symbol Re. Its atomic number (number of protons in its nucleus) is 75. Its relative atomic mass is 186.207. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Rhenium melts at 3180 °C and boils at 5627 °C. Chemists classify rhenium as a transition metal . For information on the position of rhenium on the periodic table, see the article Periodic table .
The German scientists Walter Noddack, Ida Tacke, and Otto Berg discovered rhenium in 1925.
