Salt is a clear, brittle mineral used to flavor and preserve food. People have used salt since ancient times. Today, salt is also used in the manufacture of chemicals and chemical products.
Salt consists of the elements sodium and chlorine. Its chemical name is sodium chloride. Its formula is NaCl. Its mineral name is halite. Salt usually forms clear crystals that are almost perfect cubes. However, impurities in the salt may make it appear to be white, gray, yellow, or red. Table salt also appears white. But it actually consists of clear cubes.

The source of all salt is brine (salty water) from seas, salt lakes, and similar bodies of water. Salt deposits that now lie underground were formed by the evaporation of seawater millions of years ago.
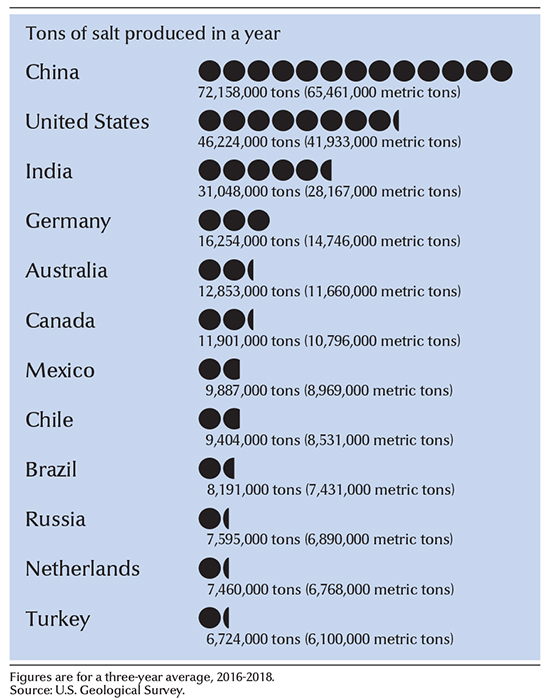
China and the United States rank as the leading countries in salt production. Other important salt-producing countries include Australia, Brazil, Canada, Chile, Germany, India, and Mexico.

Salt is necessary to good health. Human blood contains salt. Body cells must have salt to function properly. However, some studies have suggested that too much salt or other sodium compounds in a person’s diet can lead to high blood pressure. For this reason, many people attempt to reduce the amount of salt they eat. Some people use salt substitutes that do not contain sodium.
Uses of salt
In the chemical industry.
The chemical industry consumes the largest amounts of salt. The industry uses salt mainly to produce other chemicals. Salt can be broken down and used to make a variety of sodium and chlorine products. About 20 percent of the salt consumed in the United States is used to make a sodium compound called soda or soda ash. Soda is used primarily in the manufacture of glass and soap.
Chlorine products account for about 45 percent of the salt consumed in the United States each year. Chlorine compounds are used in the manufacture of paper, plastics, pesticides, cleaning fluids, and antifreeze and other automotive fluids.
As a deicer.
When salt is mixed with ice, the melting point of ice is lowered. As a result, salt is often spread on roads and highways to melt snow and ice. About 20 percent of the salt consumed in the United States is used for as road salt.
In the food industry.
Only about 5 percent of the salt that is consumed in the United States today is used as a seasoning. This figure includes both use of salt in the home and industrial use by food-processing plants.
Other uses.
Salt is also used in a wide range of other products and processes. These uses include ceramic glazes, medicines, oil refining, refrigeration, sewage treatment, textile dyes, and water softening.
Where salt comes from
Salt from the sea.
Seawater is salty because rain water dissolves minerals containing sodium and chlorine in rocks and soil. Rivers carry these dissolved minerals to the sea. Evaporating seawater is the oldest method of obtaining salt. Salt that comes from evaporated seawater is often called solar salt.
Seawater consists of about 2.5 percent salt and about 1 per cent other minerals. The other minerals are mostly compounds of calcium, potassium, and magnesium. In a commercial solar salt operation, salt is obtained from seawater by moving the water through a series of evaporating ponds. The various minerals in seawater precipitate (separate from the water) at different rates. Most of the other minerals precipitate before salt does. They are thus left behind as the seawater is moved from one pond to another. Most salt that is produced by this evaporation technique is 95 to 98 percent pure sodium chloride.

Solar saltworks require a hot, dry climate to hasten evaporation. Much solar salt is produced in Australia, the Bahamas, China, India, Mexico, and near the Dead and Mediterranean seas. In the United States, solar salt is produced near Utah’s Great Salt Lake, and in other southwestern states.
Salt from the ground.
Salt that occurs in hard, massive layers beneath the ground is called rock salt. These deposits were formed by the evaporation of large parts of oceans millions of years ago. They occur along with deposits of calcium carbonate and potash, minerals that are also found in seawater.

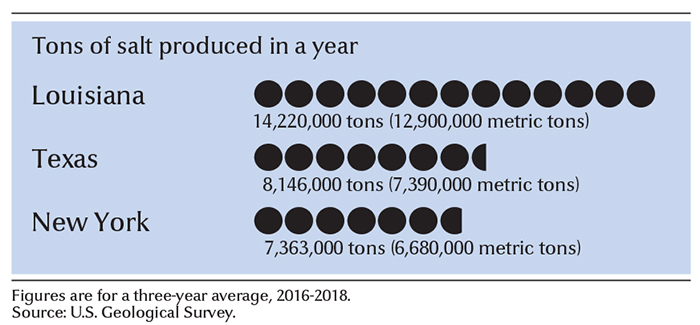
Underground salt deposits are found on every continent. In the United States, these deposits occur in 32 states. Some of the largest underground salt deposits in the United States are the Salina Basin salts, in Michigan, Ohio, New York, Pennsylvania, and West Virginia. Other major deposits are the Permian Basin salts, in Kansas, Oklahoma, Texas, and New Mexico; and the Gulf Coast salts, in Alabama, Mississippi, Louisiana, and Texas. Most of the salt mined in Canada comes from underground deposits in southwestern Ontario.
Most of the Gulf Coast salt deposits occur in formations called salt domes. Salt is lighter than most other minerals and will flow when it is under great pressure. Salt domes are formed when beds of rock salt flow upward and break through overlying rock.
There are two basic methods for removing salt from the ground—room-and-pillar mining and solution mining. In room-and-pillar mining, shafts are sunk into the ground. Miners break up the rock salt with drills. The miners remove chunks of salt, creating huge rooms separated by pillars of salt. The room-and-pillar method requires that about half of the salt be left behind as pillars. Room-and-pillar mining is also used in mining coal. For more information about room-and-pillar mining, see Coal (Underground mining).
In solution mining, a well is drilled into the ground. Two pipes are lowered into the hole. The pipes consist of a small central pipe inside a larger pipe. Fresh water is pumped down the central pipe to the salt deposit. The water dissolves some salt to form brine. The brine is then pumped to the surface through the outer pipe. The brine is either shipped as a liquid or evaporated in special devices called vacuum pans to form solid salt.
How table salt is made
After salt has been obtained by mining or evaporation, it is sorted for quality. Then it is crushed, ground, and screened into batches according to particle size. High-quality salt that has been ground into fine particles is used as table salt. This fine-grained salt tends to cake at high humidities. For this reason, manufacturers add a free-flowing agent (substance that prevents caking) to it before packaging. Common free-flowing agents include magnesium carbonate, calcium carbonate, calcium silicate, and calcium phosphate. All of these compounds are colorless, odorless, tasteless, and harmless.
Much of the table salt purchased by consumers is also iodized. That means it has potassium iodide or sodium iodide added to it. A lack of iodine in a person’s diet can result in a condition called goiter, in which the thyroid gland becomes enlarged. A small amount of iodine is enough to prevent goiter. The addition of iodine compounds to table salt enables large numbers of people to get the iodine they need.
History of salt
Salt has been a precious commodity since ancient times. It was often traded ounce for ounce for gold. The early Chinese used coins made of salt for currency. In many areas around the Mediterranean Sea, salt cakes were used as currency. Several ancient cultures also levied taxes on salt.
The main sources of salt in ancient times were dry coastal areas, particularly those surrounding the Mediterranean Sea. The earliest trade routes centered on Spain, Italy, Greece, and Egypt. Many of the first roads and caravan routes were established for the purpose of transporting salt. Cities such as Genoa, Pisa, and Venice developed as centers for the salt trade.
In the 1300’s, people near the coast of the North Sea in northern Europe began trading for salt. They needed it to preserve fish for shipping to inland markets. Later, salt was obtained by boiling the brine from salt springs. Many cities and towns grew up near such springs throughout Europe.
Boiling brine to obtain salt required large supplies of wood for fuel. This problem was partly solved in the 1700’s after coal began to be widely used. England became the largest producer of salt in the world at that time, partly because of its abundant supply of coal.
Colonial America received most of its salt from England. After the American Revolution (1775-1783), saltworks were set up along the Atlantic Coast for boiling seawater. A salt industry also grew up near where the city of Syracuse, New York, now stands. Salt springs were discovered there. The need to transport salt from Syracuse was one of the main reasons for the construction of the Erie Canal, completed in 1825.
Deep-drilling technology was introduced into salt production in the early 1800’s. Most early wells were drilled to improve the quality of salt springs that were already being used in salt production and to locate new ones. Production of salt from underground mines began in the mid-1800’s. Drilling helped revolutionize knowledge of Earth’s layers. This new knowledge led to increased exploration for salt, potash, and petroleum. Salt exploration indirectly gave rise to such industries as the potash industry and the petroleum industry.
Today, the U.S. government is investigating the possibility of storing radioactive wastes in underground salt mines. Salt mines have several characteristics that make them good sites for radioactive wastes. For example, they have remained stable and dry for millions of years. Most salt mines occur in areas where earthquakes are rare. Salt also is capable of absorbing heat from surrounding objects. It also flows to seal up fractures that form in the walls.
