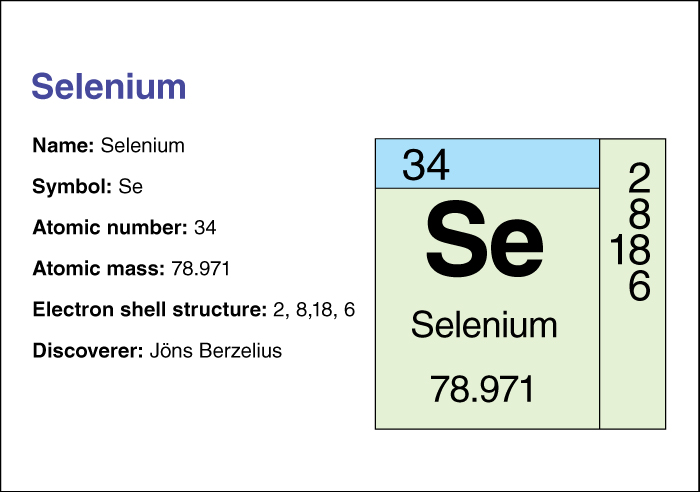
Selenium, << sih LEE nee uhm, >> is a nonmetallic chemical element. In nature, it most often occurs in combination with such metals as copper, lead, and silver. The Swedish chemist Jons J. Berzelius first isolated selenium in 1817. Human beings and other animals require tiny amounts of selenium in their diet. The element helps the body convert fats and protein into energy. Foods that supply selenium include meat and fish.
Selenium exists in several forms called allotropes. The most stable allotrope is gray selenium, a solid that forms when the element is heated to 180 °C and cooled. Gray selenium exhibits some metalloid characteristics, such as being a semiconductor. Gray selenium becomes a better conductor of electric current when exposed to light and heat. For these reasons, it is used to make electric eyes, solar batteries, and photographic exposure meters. It also is used in electrostatic copying machines.
Element, Chemical (table: Table of the elements)
Selenium has the symbol Se. Its atomic number (number of protons in its nucleus) is 34. Its relative atomic mass is 78.96. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Gray selenium melts at 217 °C and boils at 685 °C. For information on the position of selenium on the periodic table, see the article Periodic table .
