Sodium is a silvery-white metallic element that has many important uses. It is a soft metal, and can easily be molded or cut with a knife. Sodium belongs to a group of chemical elements that are called the alkali metals.
Where sodium is found.
Sodium is the sixth most common chemical element in the earth’s crust. It makes up about 2.8 percent of the crust. Sodium never occurs pure—that is, as a separate element—in nature. It combines with many other elements, forming compounds. To obtain pure sodium, the metal must be extracted (removed) from its compounds.
One of the most familiar sodium compounds is sodium chloride, which is common table salt. Sodium chloride can be found in dry lake beds, underground, and in seawater. Countries with large deposits of sodium chloride include Belarus, China, France, Germany, India, Russia, Ukraine, the United Kingdom, and the United States.
Such minerals as borax and cryolite contain sodium. Many plants and the bodies of animals contain small amounts of sodium. The human body needs a certain amount of sodium to maintain a normal flow of water between the body fluids and the cells. Sodium also plays a part in tissue formation and muscle contraction.
A number of studies have shown that the foods in a balanced diet contain enough sodium for the body’s normal needs, without the addition of table salt. In fact, some studies have indicated that too much sodium in a person’s diet can lead to high blood pressure.
Uses.
Sodium compounds have many uses in industry, medicine, agriculture, and photography. Manufacturers use sodium borate (borax) in making ceramics, soaps, water softeners, and many other products (see Borax). Sodium hydroxide (caustic soda, or lye) is an important industrial alkali used in refining petroleum and in making paper, soaps, and textiles. Sodium carbonate (soda ash or washing soda) is used in the manufacture of sodium bicarbonate (baking soda). Many people take sodium bicarbonate to relieve an overly acid stomach. Sodium nitrate (Chile saltpeter) is a valuable fertilizer. Photographers use sodium thiosulfate (hypo) to fix photographic images on paper.
Pure sodium also has industrial uses. Finely divided sodium is used as a catalyst (substance that causes a chemical reaction) in the manufacture of some types of synthetic rubber. Some nuclear power plants use sodium in liquid form to cool nuclear reactors. Sodium is also used to produce such metals as titanium and zirconium.
Extracting sodium.
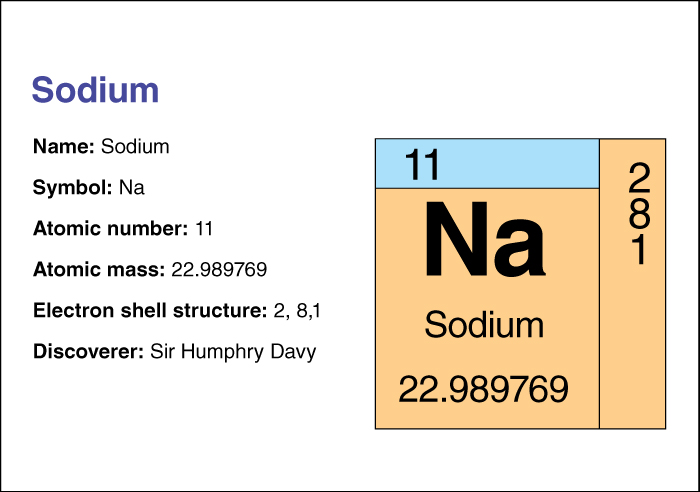
In 1807, the English chemist Sir Humphry Davy became the first person to obtain pure sodium. He used electric current to extract the metal from sodium hydroxide.
Manufacturers still use electric current to obtain sodium. The process is called electrolysis. In this process, a current is passed through a molten sodium compound, such as sodium chloride. The current separates the compound into chlorine gas and sodium metal. See Electrolysis.
Chemical properties.
Pure sodium is extremely active chemically. Sodium immediately combines with oxygen when it is exposed to the air. As a result, the element loses its shiny appearance and becomes dull. Sodium’s bright surface can be seen only after it has been newly cut or extracted.
Sodium weighs less than water. It decomposes (breaks up) water, producing hydrogen gas and sodium hydroxide. This chemical reaction is extremely violent. It produces much heat that often causes the hydrogen to ignite.
The element also reacts quickly with such other nonmetals as chlorine and fluorine, and it forms alloys with many metals. Liquid ammonia dissolves sodium, forming a dark-blue solution. A test for determining whether a material contains sodium is to hold the substance in a flame. If sodium is present, the flame will burn a bright yellow.
Sodium must be handled and stored with extreme care. In laboratories, small amounts are stored under kerosene in airtight bottles. The kerosene prevents air or moisture from reaching the metal. Large quantities of sodium in brick form are stored and shipped in airtight, moisture-free barrels. Sodium is also shipped in sealed tank cars. The metal is melted and poured into the tanks. The sodium hardens during shipping, and must be melted again before it can be removed.
Sodium has the chemical symbol Na. Its atomic number (number of protons in its nucleus) is 11. Its relative atomic mass is 22.989770. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of an atom of carbon 12, the most abundant form of carbon. The melting point of sodium is 97.8 °C, and its boiling point is 881 °C.
Chemists classify sodium as an alkali metal. For information on the position of sodium on the peroidic table, see the article Periodic table.
