Tantalum << TAN tuh luhm >> is a hard, shiny, silver-colored metallic element. Its chemical symbol is Ta. Pure tantalum metal is ductile (easily shaped). It serves as an important element in many strong alloys (combinations of two or more metals). Engineers use tantalum alloys to make aircraft and missile parts and to build devices called nuclear reactors that produce energy. Doctors and dentists use tantalum in surgical instruments and other medical devices because the metal does not react with body fluids.
At normal temperatures, a film of tantalum oxide (Ta2O5) forms on the surface of tantalum, protecting the metal from corrosion (chemical breakdown). Tantalum oxide serves as a good insulator—that is, it does not conduct electric current well. For this reason, electronics manufacturers use tantalum in devices called capacitors that store an electric charge. Camera manufacturers add tantalum oxide to glass to increase the refracting (light-bending) power of camera lenses.
Tantalum is a relatively rare element in nature. Significant concentrations of the element appear in the minerals columbite and tantalite. These minerals contain both tantalum and niobium, which are chemically similar and therefore difficult to separate from each other. Manufacturers obtain tantalum for commercial use as a by-product in the production of tin. Leading tantalum-producing countries include Australia, Brazil, Burundi, China, the Democratic Republic of the Congo, Ethiopia, and Rwanda.
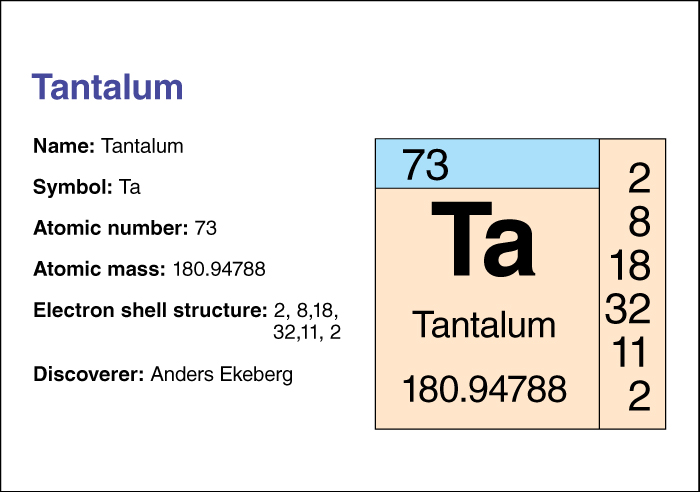
Tantalum’s atomic number (number of protons in its nucleus) is 73. Its relative atomic mass is 180.9479. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most stable isotope of carbon. Tantalum has high melting and boiling points. It melts at 3017 °C and boils at around 5458 °C. Its density is 16.4 grams per cubic centimeter.
Chemists classify tantalum as a transition metal. For information on the position of bromine on the periodic table, see the article Periodic table.
Tantalum was discovered in 1802 by the Swedish chemist Anders G. Ekeberg.
