Thulium, << THOO lee uhm, >> is one of the lanthanide elements. It occurs with other lanthanide elements in such minerals as gadolinite, euxenite, and xenotime. Portable X-ray units use radioactive thulium. Such units require no electrical equipment, and they need to be recharged with thulium only once every few months. The Swedish scientist Per Cleve discovered thulium in 1879. The element’s name comes from Thule, the Latin word for the northernmost part of the inhabitable world.
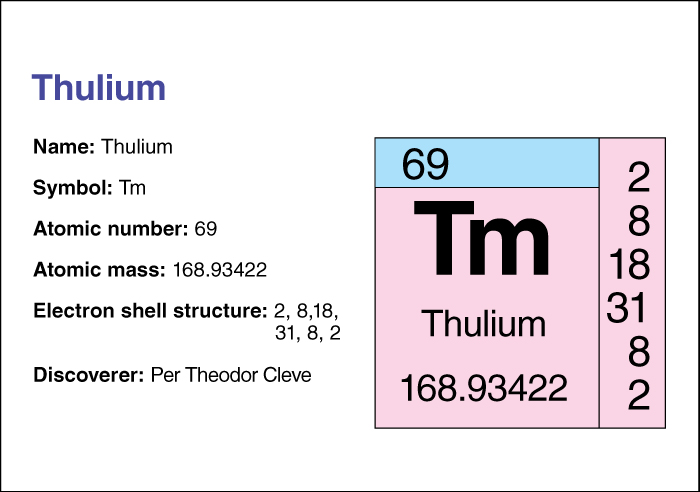
Thulium’s chemical symbol is Tm. Its atomic number (number of protons in its nucleus) is 69. Its relative atomic mass is 168.93421. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Thulium melts at 1545 °C and boils at 1950 °C. It has a density of 9.321 grams per cubic centimeter at 25 °C. For information on the position of thulium on the periodic table, see the article Periodic table .
See also Rare earth. .
