Yttrium << IHT ree uhm >> is a silvery-white metallic element . It has a number of important uses, particularly in the electronics industry. For example, yttrium compounds help produce light in devices called light-emitting diodes (LED’s). The compound yttrium oxide is used to produce two kinds of crystals called garnets. One type of garnet acts as a microwave filter in radar, and the other serves as an imitation diamond. Yttrium is also used in lasers and in the manufacture of certain chemicals, glass, and ceramics.
Yttrium resembles the lanthanide elements, and it occurs in nearly all lanthanide minerals. The metal is obtained commercially from monazite sand.
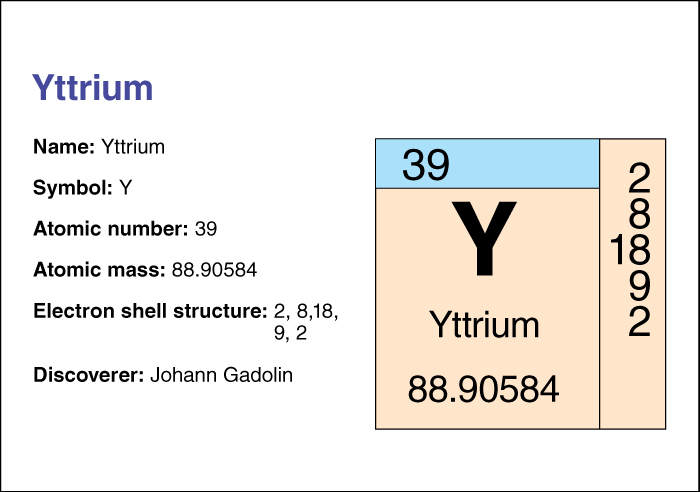
Yttrium has the chemical symbol Y. Its atomic number (number of protons in its nucleus) is 39. Its relative atomic mass is 88.90585. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Yttrium melts at 1522 °C (plus or minus 8 °C) and boils at 3338 °C. The Swedish chemist Carl Gustav Mosander discovered yttrium in 1843 in the mineral yttria. Yttrium belongs to the group of elements called transition metals. For information on the position of yttrium on the periodic table, see the article Periodic table .
